1. Choose the correct option
(A) Which of the following methods can be used to seperate two compounds with different solubilities in the same solvent?
(a) Fractional crystallization
(b) Crystallization
(c) Distillation
(d) Solvent extraction
(B) Which of the following techniques is used for seperation of glycerol from soap in soap industry ?
(a) Distillation under reduced pressure
(b) Fractional distillation
(c) Filtration
(d) Crystallization
(C) Which technique is widely used in industry to seperate components of mixture and also to purify them ?
(a) Steam distillation
(b) Chromatography
(c) Solvent extraction
(d) Filtration
(D) A mixture of acetone and benzene can be seperated by the following method ______ .
(a) Simple distillation
(b) Fractional distillation
(c) Distillation under reduced pressure
(d) Sublimation
(E) Colourless components on chromatogram can not be observed by the following :
(a) Using UV light
(b) Using iodine chamber
(c) Using the spraying reagent
(d) Using infrared light
2. Answer the following
(A) Which of the following techniques is used for purification of solid organic compounds?
(a) Crystallisation (b) Distillation
Ans: Solid (crude/impure) organic compounds can be purified by crystallization
(B) What do you understand by the terms
(a) residue (b) filtrate.
Ans:
(a) Residue: In the process o f filtration, the insoluble (undissolved) impurities which remain on the filter paper are called residue.
(b )Filtrate: In the process o f filtration, the liquid which pass through the filter paper and collected in the beaker is calledfiltrate.
(C) Why is a condenser used in distillation process?
Ans: Before filtration, filter paper is moistened with appropriate solvent to ensure that it sticks to the funnel and does not let the air to pass through the leaks.
(D) Why is paper moistened before filtration?
Ans: Before filtration, filter paper is moistened with appropriate solvent to ensure that it sticks to the funnel and does not let the air to pass through the leaks.
(E) What is the stationary phase in Paper Chromatography?
Ans: Paper chromatography is a type of partition chromatography in which a special quality paper, namely Whatman paper 1 is used. The water trapped in the fibres of the paper acts as stationary phase.
(F) What will happen if the upper outlet of the condenser is connected to the tap instead of the lower outlet?
Ans:
(a) If water enters through upper outlet of condenser, the water will quickly flow down under the influence of gravity. This allows only a small section of the condenser to be cooled enough.
(b) If water enters through lower outlet of condenser, the entire condenser will be tilled with water before it leaves out providing maximum cooling to the condenser. This results in maximum recovery of purified liquid.
Hence, water must be allowed to enter through lower outlet of condenser during distillation process.
(G) Give names of two materials used as stationary phase in chromatography.
Ans : (a) Alumina (b) Silica gel
(H) Which properties of solvents are useful for solvent extraction?
Ans: (a) Organic compound must be more soluble in the organic solvent, than in water.
(b) Solvent should be immiscible with water and be able to form two distinct layers.
(I) Why should spotting of mixture be done above the level of mobile phase ?
Ans: (a) If spotting of a mixture is done at the level of mobile phase, then solvent will come in contact with the sample spot.
(b) Sample spot will dissolve in the mobile phase and its components will move all over the plate resulting in no distinct separation.
Hence, spotting of mixture should be done above the level of mobile phase.
(J) Define : (a) Stationary phase (b) Saturated solution
Ans:
(a) Stationary phase : Stationary phase is a solid or a liquid supported on a solid which remains fixed in a place and on which different solutes are adsorbed to different extent.
(b) Saturated solution : A saturated solution is a solution which cannot dissolve additional quantity o f a solute.
(K) What is the difference between simple distillation and fractional distillation?
Ans : 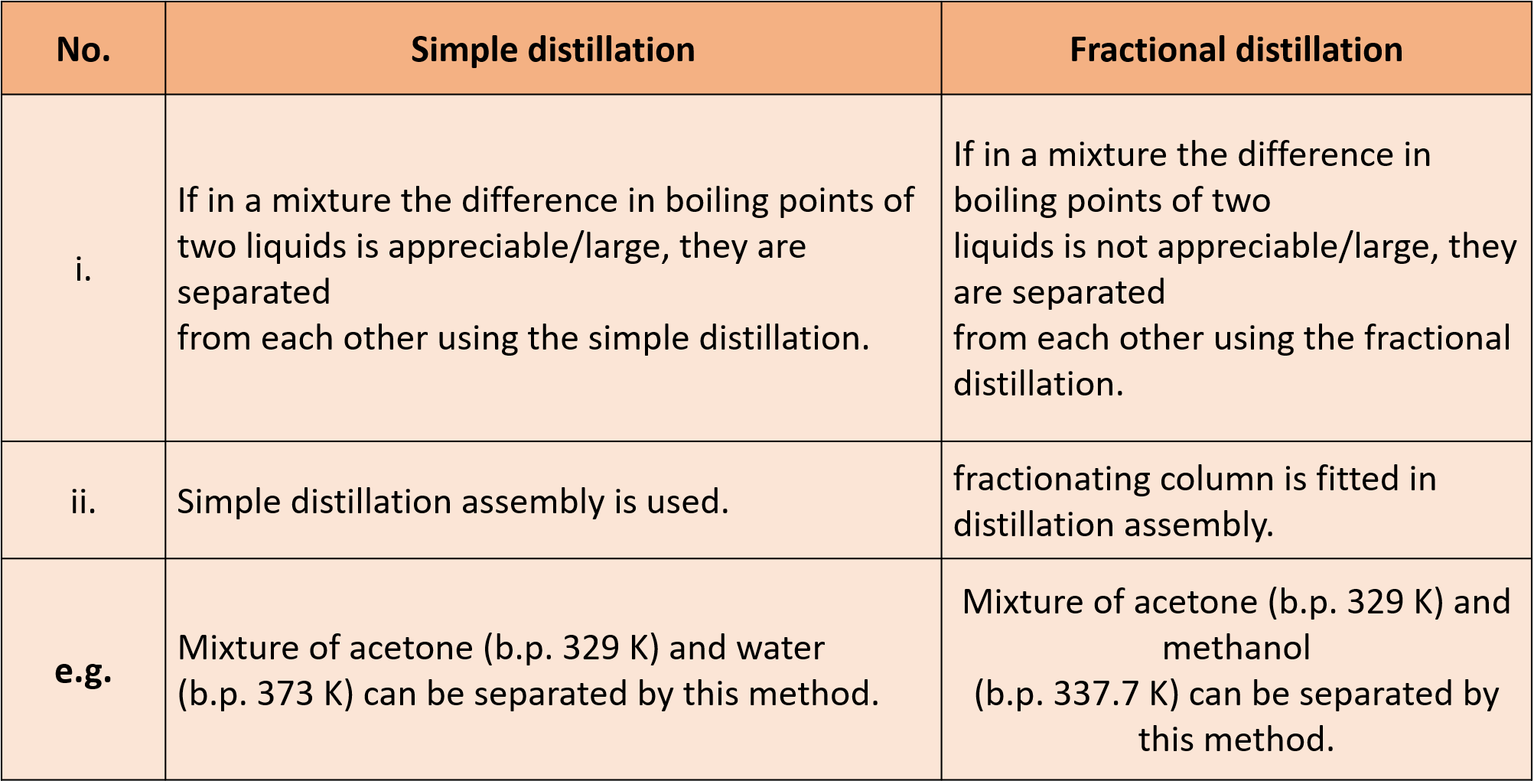
(L) Define : (a) Solvent extraction (b) Distillation.
Ans:
(a) Solvent extraction : Solvent extraction is a method used to separate an organic compound present in an aqueous solution, by shaking it with a suitable organic solvent in which the compound is more soluble than water.
(b) Distillation : The process in which liquid is converted into its vapour phase at its boiling point and the vapour is then condensed back to liquid on cooling is known as distillation.
(M) List the properties of solvents which make them suitable for crystallization.
Ans: The solvent to be used for crystallization should have following properties:
(a) The compound to be crystallized should be least or sparingly soluble in the solvent at room temperature but highly soluble at high temperature.
(b) Solvent should not react chemically with the compound to be purified.
(c) Solvent should be volatile so that it can be removed easily.
(N) Name the different types of Chromatography and explain the principles underlying them.
Ans : Depending on the nature of the stationary phase i.e., whether it is a solid or a liquid, chromatography is classified into adsorption chromatography and partition chromatography.
(a) Adsorption chromatography : This technique is based on the principle of differential adsorption. Different solutes are adsorbed on an adsorbent to different extent. Adsorption chromatography is further classified into two types:
(i) Column chromatography (ii) Thin-layer chromatography
(b) Partition chromatography : This technique is based on continuous differential partitioning of components of a mixture between stationary and mobile phases. For example, paper chromatography.
(O) Why do we see bands separating in column chromatography?
Ans: (a) In column chromatography, the solutes get adsorbed on the stationary phase and depending on the degree to which they are adsorbed, they get separated from each other.
(b) The component which is readily adsorbed are retained on the column and others move down the column to various distances forming distinct bands.
Hence, we see bands separating in column chromatography.
(P) How do you visualize colourless compounds after separation in TLC and Paper Chromatography?
Ans: (a) Thin-laver chromatography (TLC): If components are colourless but have the property of tluorescence
then they can be visualized under UV light, or the plate can be kept in a chamber containing a few iodine
crystals. The iodine vapours are adsorbed by the components and the spots appear brown. Also, spraying
agent like ninhydrin can also be used (for amino acids).
(b) Paper Chromatography: The spots of the separated colourless components may be observed either
under ultra-violet light or by the use of an appropriate spraying agent.
(Q) Compare TLC and Paper Chromatography techniques.
Ans : 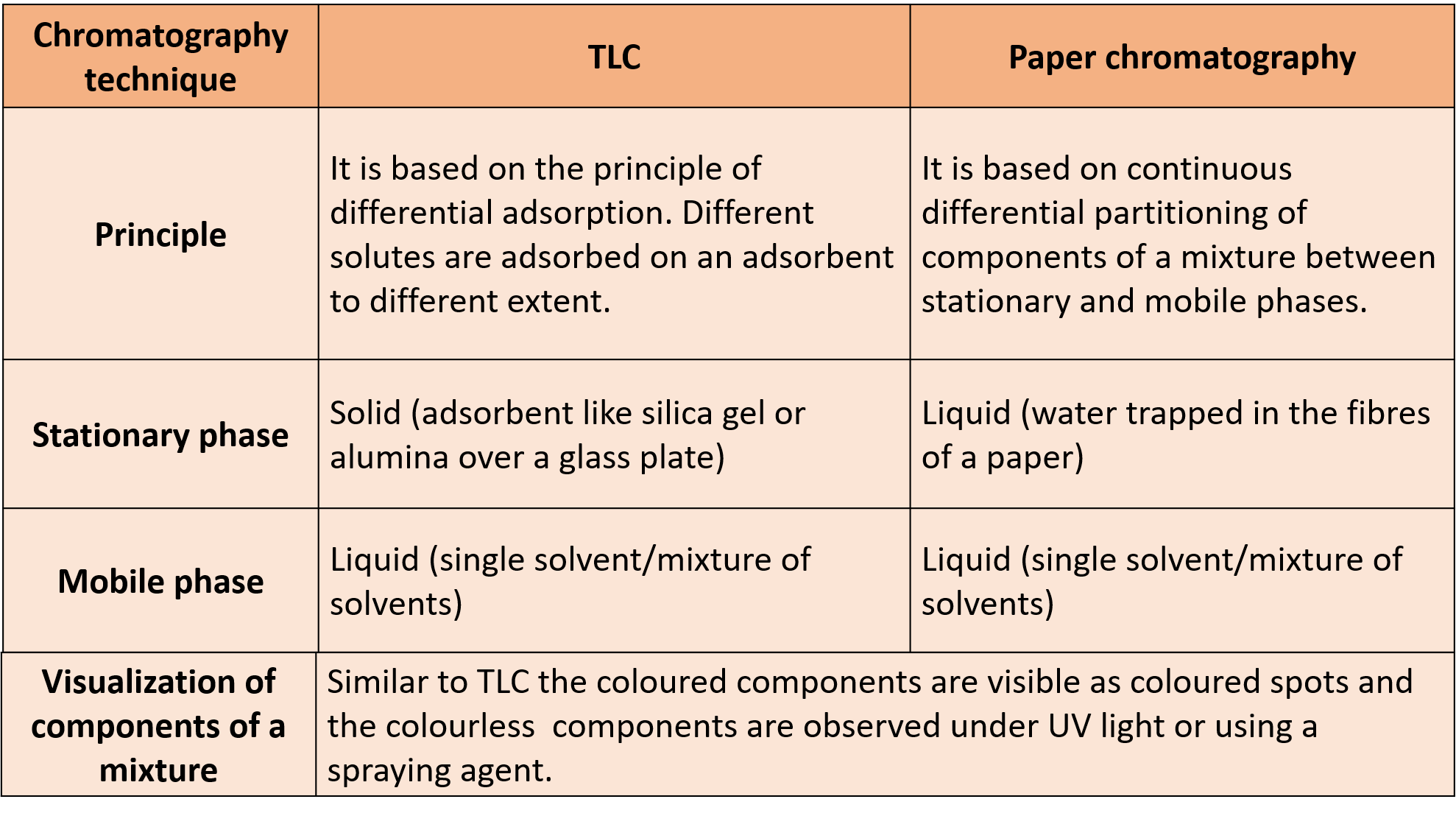
3. Label the diagram and explain the process in your words.
Ans :
(i) When filtration is carried out using a vacuum pump it is called filtration under suction. It is a faster and
more efficient technique than simple filtration. The diagram is as follows:
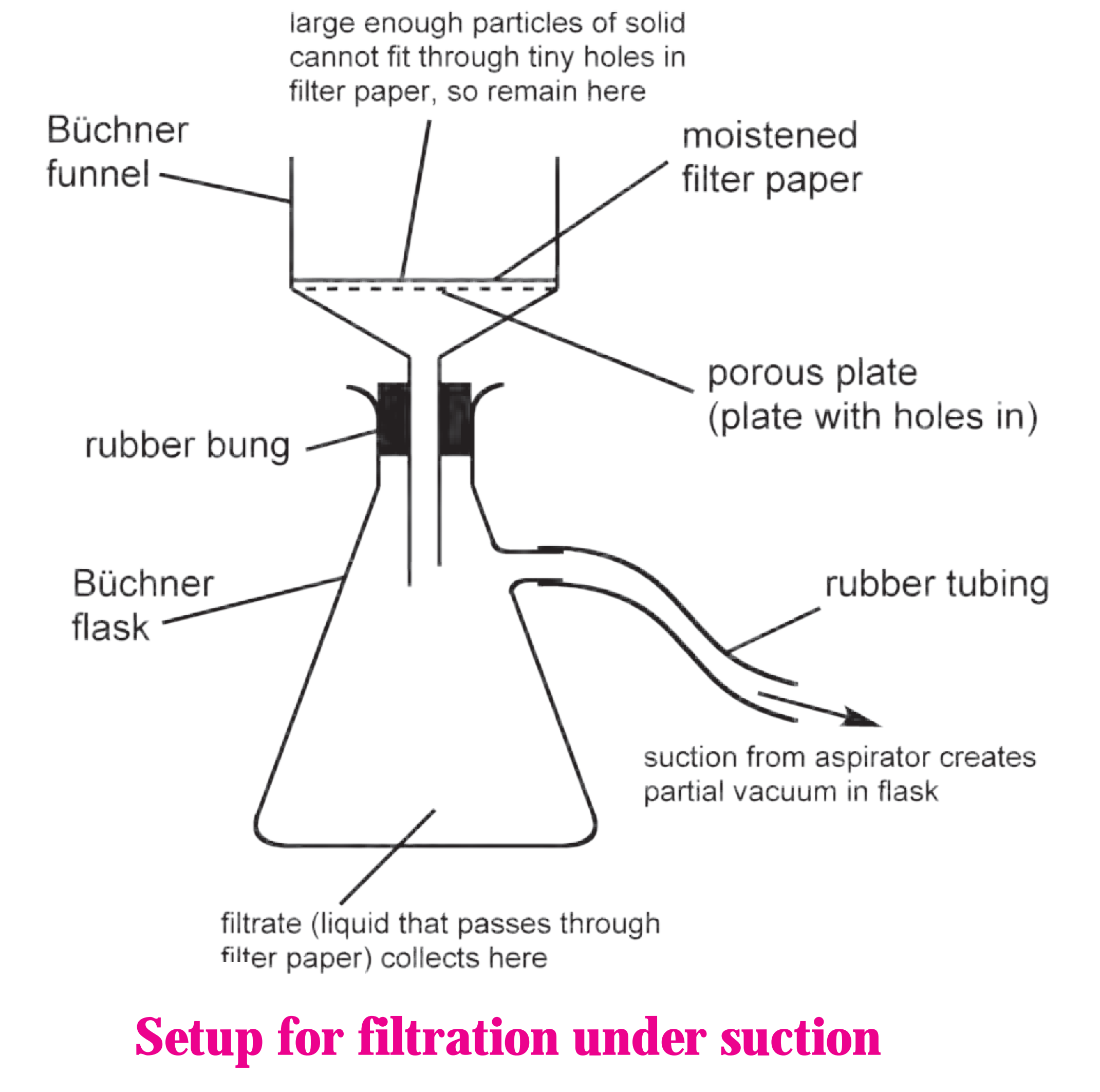
(ii) Procedure:
(a) The assembly for filtration under suction consists of a thick wall conical flask with a side arm (Buchner flask).
(b) The flask is connected to a safety bottle by rubber tube through the side arm.
(c) Buchner funnel (a special porcelain funnel with a porous circular bottom) is fitted on the conical flask with the help of a rubber cork.
(d) A circular filter paper of correct size is placed on the circular porous bottom of the Buchner funnel and the funnel is placed on the flask.
(e) Filter paper is moistened with a few drops of water or solvent.
(f) Suction is created by starting the pump and filtration is carried out.
(iii) Crystals are collected on the filter paper and filtrate in the flask.