1. Choose correct option
A. The branch of chemistry which deals with study of separation, identification, and quantitative determination of the composition of different substances is called as …………
a. Physical chemistry
b. Inorganic chemistry
c. Organic chemistry
d. Analytical chemistry
B. Which one of the following property of matter is Not quantitative in nature ?
a. Mass
b. Length
c. Colour
d. Volume
C. SI unit of mass is ……..
a. kg
b. mol
c. pound
d. m3
D. The number of significant figures in 1.50 × 104 g is ………..
a. 2
b. 3
c. 4
d. 6
E. In Avogadro’s constant 6.022 × 104 mol-1, the number of significant figures is ……….
a. 3
b. 4
c. 5
d. 6
F. By decomposition of 25 g of CaCO3, the amount of CaO produced will be …………
a. 2.8 g
b. 8.4 g
c. 14.0 g
d. 28.0 g
G. How many grams of water will be produced by complete combustion of 12 g of methane gas
a. 16
b. 27
c. 36
d. 56
H. Two elements A (At. mass 75) and B (At. mass 16) combine to give a compound having 75.8 % of A. The formula of the compound is
a. AB
b. A2B
c. AB2
d. A2B3
I. The hydrocarbon contains 79.87 % carbon and 20.13 % of hydrogen. What is its empirical formula ?
a. CH
b. CH2
c. CH2
d. C2H2
J. How many grams of oxygen will be required to react completely with 27 g of Al? (Atomic mass : Al = 27, O = 16)
a. 8
b. 16
c. 24
d. 32
K. In CuSO 4.5 H2O the percentage of water is …………. (Cu = 63.5, S = 32, O = 16, H = 1)
a. 10 %
b. 36 %
c. 60 %
d. 72 %
L. When two properties of a system are mathematically related to each other, the relation can be deduced by
a. Working out mean deviation
b. Plotting a graph
c. Calculating relative error
d. all the above three
2. Answer the following questions
A. Define : Least count
Ans: The smallest quantity that can be measured by the measuring equipment is called least count.
B. What do you mean by significant figures? State the rules for deciding significant figures.
Ans: i. The significant figures in a measurement or result are the number of digits known with certainty plus one uncertain digit.
ii. Rules for deciding significant figures:
(a) All non-zero digits are significant.
e.g. 127.34 g contains five significant figures which are 1, 2, 7, 3 and 4.
(b) All zeros between two non-zero digits are significant,
e.g. 120.007 m contains six significant figures.
(c) Zeros on the left of the first non-zero digit are not significant. Such a zero indicates the position of the decimal point.
e.g. 0.025 has two significant figures, 0.005 has one significant figure.
(d) Zeros at the end of a number arc significant if they arc on the right side of the decimal point,
e.g. 0.400 g has three significant figures and 400 g has one significant figure.
(e) In numbers written is scientific notation, all digits are significant.
e.g. 2.035 x 102 has four significant figures and 3.25 x 105 has three significant figures.
C. Distinguish between accuracy and precision.
Ans: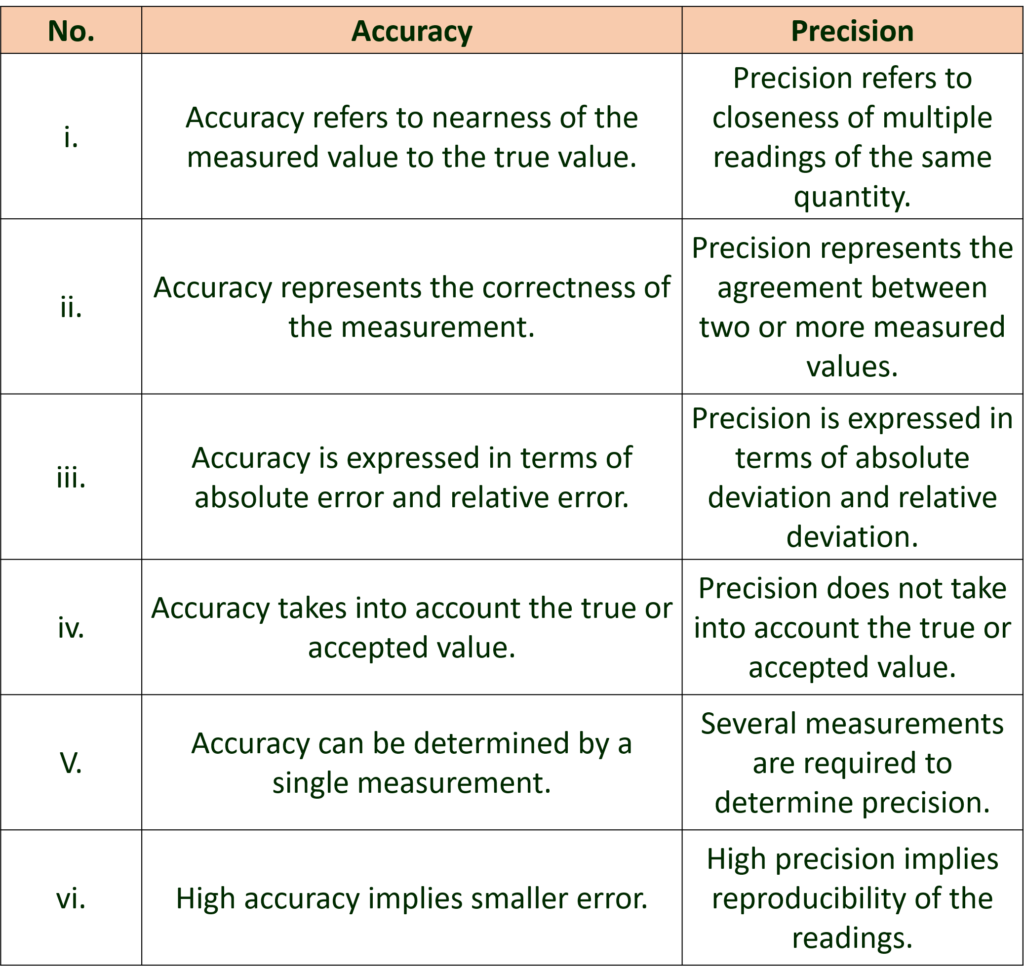
D. Explain the terms percentage composition, empirical formula and molecular formula.
Ans : i. Percentage composition:
(a) The percentage composition of a compound is the percentage by weight of each element present in the compound.
(b) Quantitative determination of the constituent elements by suitable methods provides the percent elemental composition of a compound.
(c) If the percent total is not 100, the difference is considered as percent oxygen.
(d) From the percentage composition, the ratio of the atoms of the constituent elements in the molecule is calculated.
ii. Empirical formula: The simplest ratio o atoms of the constituent elements in a molecule is called the empirical formula of that compound.
e. g. The empirical formula of benzene is CH.
iii. Molecular formula;
(a) Molecular formula of a compound is the formula which indicates the actual number of atoms of the constituent elements in a molecule.
e.g. The molecular formula of benzene is C6H6–
(b) It can be obtained from the experimentally determined values of percent elemental composition and molar mass of that compound.
(c) Molecular formula can be obtained from the empirical formula if the molar mass is known.
Molecular formula = r x Empirical formula
E. What is a limiting reagent ? Explain.
Ans: (a). The reactant which gets consumed and limits the amount o f product formed is called the limiting reagent.
(b). When a chemist carries out a reaction, the reactants are not usually present in exact stoichiometric amounts, that is, in the proportions indicated by the balanced equation.
(c). This is because the goal of a reaction is to produce the maximum quantity of a useful compound from the starting materials. Frequently, a large excess of one reactant is supplied to ensure that the more expensive
reactant is completely converted into the desired product.
(d). The reactant which is present in lesser amount gets consumed after some time and subsequently, no further reaction takes place, whatever be the amount left of the other reactant present.
Hence, limiting reagent is the reactant that gets consumed entirely and limits the reaction
F. What do you mean by SI units ? What is the SI unit of mass ?
Ans: (a). In 1960, the general conference of weights and measures proposed revised metric system, called International system of Units i.e. SI units, abbreviated from its French name.
(b). The SI unit of mass is kilogram (kg)
G. Explain the following terms
(a) Mole fraction
(b) Molarity
(c) Molality
Ans:
(a). Mole fraction: Mole fraction is the ratio of number of moles of a particular component of a solution to the total number of moles of the solution.
If a substance ‘A’ dissolves in substance ‘B’ and their number of moles are nA and nB, respectively, then the mole fraction of A and B are given as: 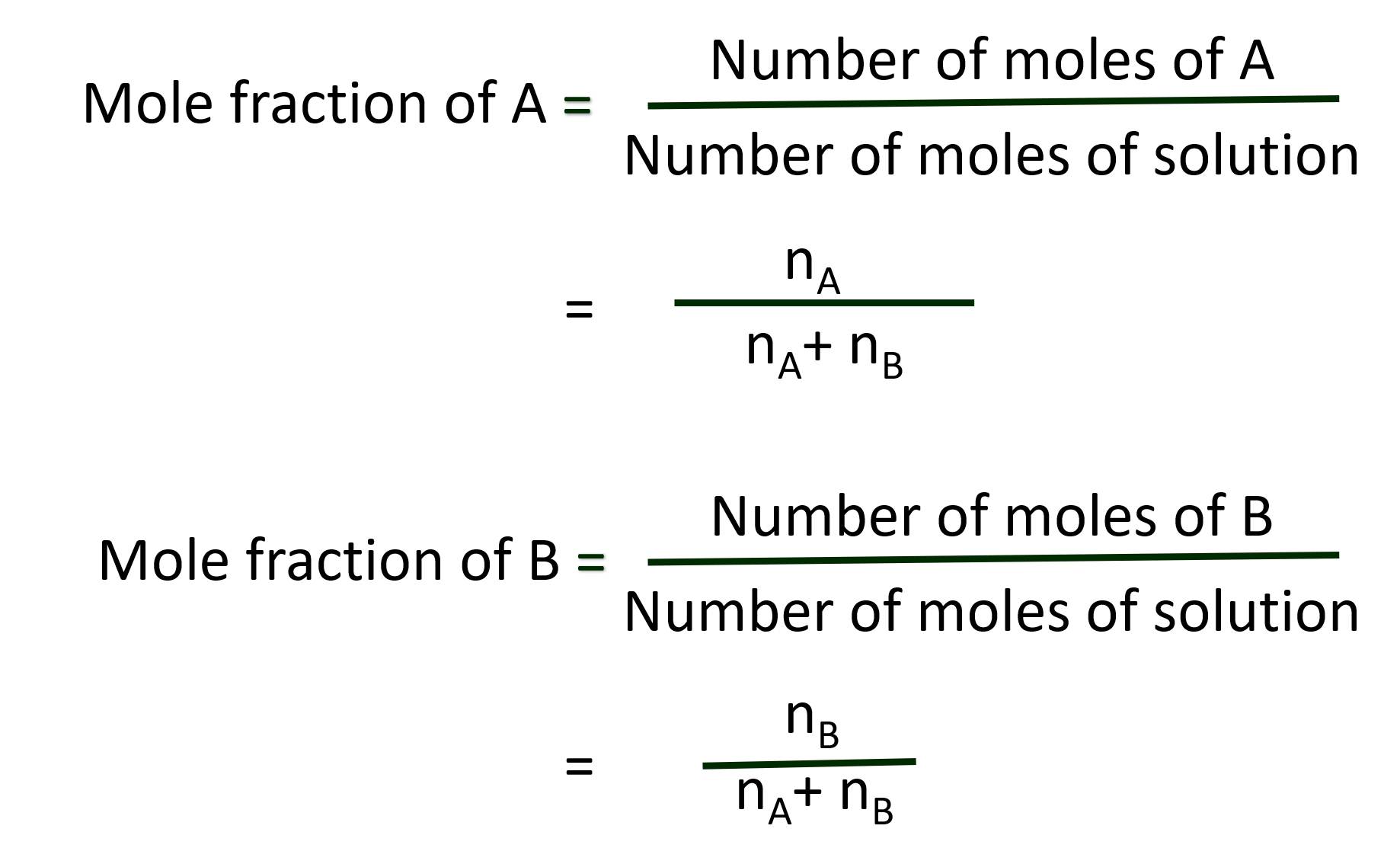
(b). Molarity: Molarity is defined as the number o f moles o f the solute present in 1 litre o f the solution. It is the most widely used unit and is denoted by M. Molarity is expressed as follows: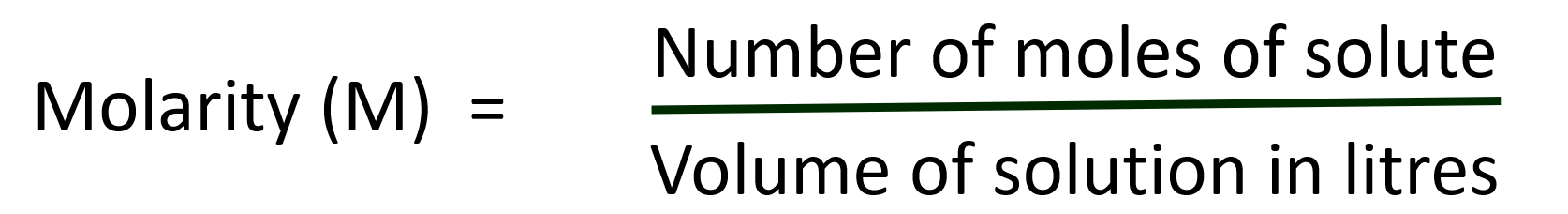
(c). Molality: Molality is the number o f moles o f solute present in 1 kg of solvent. It is denoted by m. Molality is expressed as follows:
H. Define : Stoichiometry
Ans: The study of quantitative relations between the amount of reactants and/or products is called
stoichiometry.
I. Why there is a need of rounding off figures during calculation ?
Ans:
(a). When performing calculations with measured quantities, the rule is that the accuracy of the final result is limited to the accuracy of the least accurate measurement.
(b). In other words, the final result cannot be more accurate than the least accurate number involved in the calculation.
(c). Sometimes, the final result of a calculation often contains figures that are not significant.
(d). When this occurs, the final result is rounded off.
J. Why does molarity of a solution depend upon temperature ?
Ans: (a). Molarity is the number of moles of the solute present in 1 litre of the solution. Therefore, molarity depends on the volume of the solution.
(b). Volume of the solution varies with the change in temperature.
Hence, molarity of a solution depends upon temperature.
K. Define Analytical chemistry. Why is accurate measurement crucial in science?
Ans: The branch o f chemistry which deals with the study o f separation, identification, qualitative and quantitative determination o f the compositions o f different substances, is called analytical chemistry.
(a). The accuracy of measurement is of a great concern in analytical chemistry. This is because faulty equipment, poor data processing or human error can lead to inaccurate measurements. Also, there can be intrinsic errors in the analytical measurement.
(b). When measurements are not accurate, this provides incorrect data that can lead to wrong conclusions. For example, if a laboratory experiment requires a specific amount of a chemical, then measuring the wrong amount may result in an unsafe or unexpected outcome.
(c). Hence, the numerical data obtained experimentally are treated mathematically to reach some quantitative conclusion.
(d). Also, an analytical chemist has to know how to report the quantitative analytical data, indicating the extent of the accuracy of measurement, perform the mathematical operation and properly express the quantitative error in the result.
3. Solve the following questions
A. How many significant figures are in each of the following quantities ?
a. 45.26 ft b. 0.109 in
c. 0.00025 kg d. 2.3659 × 10-8 cm
e. 52.0 cm3 f. 0.00020 kg
g. 8.50 × 104 mm h. 300.0 cg
Ans:
i. 4 ii. 3 iii. 2 iv. 5
v. 3 vi. 2 vii. 3 viii. 4
B. Round off each of the following quantities to two significant figures :
a. 25.55 mL
b. 0.00254 m
c. 1.491 × 105 mg
d. 199 g
Ans : i. 26 mL ii. 0.0025 m
iii. 1.5 x 105 mg iv. 2.0 x 102 g
C. Round off each of the following quantities to three significant figures :
a. 1.43 cm3 b. 458 × 102 cm
c. 643 cm2 d. 0.039 m
e. 6.398 × 10-3 km f. 0.0179 g
g. 79,000 m h. 42,150
i. 649.85 j. 23,642,000 mm
k. 0.0041962 kg
Ans: a. 1.43 cm3
b. 4.58 x 104 cm
c. 643 cm2 (or 6.43 x 102 cm2)
d. 0.0390 m (or 3.90 x 102 m)
e. 6.40 x 10-3 km
f. 0.0179 g (or 1.79 x 10-2 g)
g. 7.90 x 104 m
h. 4.22 x 104 (or 42,200)
i. 6.50 x 102
j. 2.36 x 107 mm
k. 0.00420 kg (or 4.20 x 10-3 kg)
D. Express the following sum to appropriate number of significant figures :
a. 2.3 × 103 mL + 4.22 × 104 mL + 9.04 × 103 mL + 8.71 × 105 mL;
b. 319.5 g – 20460 g – 0.0639 g – 45.642 g – 4.173 g
Solution:
To perform addition/subtraction operation, first the numbers are written in such a way that they have to same exponent. The coefficients are then added/subtracted.
i. (0.23 x 104 mL) + (4.22 x 104 mL) + (0.904 x 104mL) + (87.1 x 104 mL)
= (0.23 + 4.22 + 0.904 +87.1) x 104 mL
= 92.454 x 104 mL
= 9.2454 x 105 = 9.2 x 105 mL
ii. 319.5 g – 20460 g – 0.0639 g – 45.42 g – 4.173 g =-20190.3789 g
= – 20190g
Ans: i. Sum to appropriate number of significant figures = 9.2 x 105 mL
ii. Sum to appropriate number of significant figures = – 20190 g
4. Solve the following problems.
A. Express the following quantities in exponential terms.
a. 0.0003498
b. 235.4678
c. 70000.0
d. 1569.00
Ans:
i. 0.0003498 = 3.498 x 10-4
ii. 235.4678 = 2.354678 x 102
iii. 70000.0 = 7.00000 x 104
iv. 1569.00 = 1.56900 x 103
B. Give the number of significant figures in each of the following
a. 1.230 × 104
b. 0.002030
c. 1.23 × 104
d. 1.89 × 10-4
Ans:
i. 4 ii. 4 iii. 3 iv. 3
C. Express the quantities in above (B) with or without exponents as the case may be.
a. 1.230 × 104
b. 0.002030
c. 1.23 × 104b
d. 1.89 × 104
Ans:
i. 12300 ii. 2.030 x 10–3 iii. 12300 iv. 0.000189
D. Find out the molar masses of the following compounds :
a. Copper sulphate crystal (CuSO4.5H2O)
b. Sodium carbonate, decahydrate (Na2CO3.10H2O)
c. Mohr’s salt [FeSO4(NH4)2SO4.6H2O]
Solution:
a. Molar mass of CuS04.5H2O
= (1 x At. mass Cu) + (1 x At. mass S) + (9 x At. mass O) + (10 x At. mass H)
= (1 x 63.5) + (1 x 32) + (9 x 16) + (10 x 1)
= 63.5 + 32 + 144+ 10
= 249.5 g mol-1
b. Molar mass of Na2CO3.10H2O
= (2 x At. mass Na) + (1 x At. mass C) + (13 x At. mass O) + (20 x At. mass H)
= (2 x 23) + (1 x 12)+ (13 x 16)+ (20 x 1)
= 46+ 12 + 208 + 20
= 286 g mol-1
c. Molar mass of FeS04(NH4)2S04.6H2O
= (1 x At. mass Fe) + (2 * At. mass S) + (2 x At. mass N) + (14 x At. mass O) + (20 x At. mass H)
= (1 x 56) + (2 x 32) + (2 x 14) + (14 x 16) + (20 x 1)
= 56 + 64 + 28 + 224 + 20
= 392 g mol-1
Ans: a. Molar mass of C11SO4.5H2O = 249.5 g mol-1
b. Molar mass of Na2CO3.10H2O = 286 g mol-1
c. Molar mass of FeS04(NH4)2S04.6H2O = 392 g m
E. Work out the percentage composition of constituents elements in the following compounds :
a. Lead phosphate [Pb3(PO4)2],
b. Potassium dichromate (K2Cr2O7),
c. Macrocosmic salt – Sodium ammonium hydrogen phosphate, tetrahydrate (NaNH4HPO4.4H2O)
(At. mass : Pb = 207; P = 31; O = 16; K = 39; Cr = 52; Na = 23; N = 14)
Solution: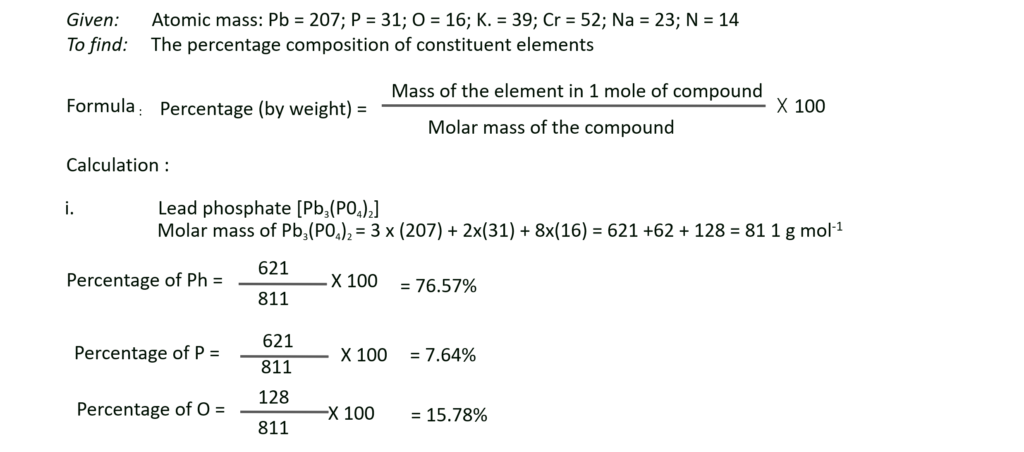
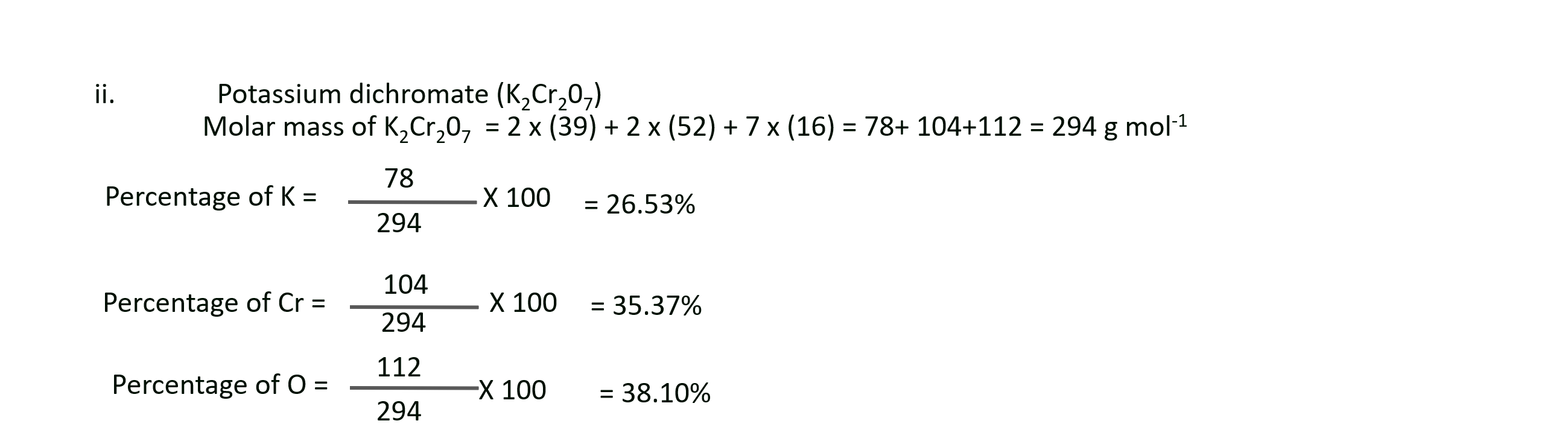
Ans: i. Mass percentage of Pb, P and O in lead phosphate [Pb3(PO4)2] are 76.57%, 7.64% and 15.78% respectively.
ii. Mass percentage of K, Cr and O in potassium dichromate (K2Cr2O7) are 26.53%, 35.37% and 38.10% respectively.
iii. Mass percentage of Na, N, P, H and O in NaNH4HP04.4H2O arc 11.00%, 6.70%, 14.83%, 6.22% and 61.24% respectively
F. Find the percentage composition of constituent green vitriol crystals (FeSO4.7H2O). Also find out the mass of iron and the water of crystallisation in 4.54 kg of the crystals. (At. mass : Fe = 56; S = 32; O = 16)
Solution :
Given: i. Atomic mass: Fe = 56; S = 32; O = 16
ii. Mass of crystal = 4.54 kg
To find: i. Mass percentage of Fe, S, H and O
ii. Mass of iron and water of crystallisation in 4.54 kg of crystal
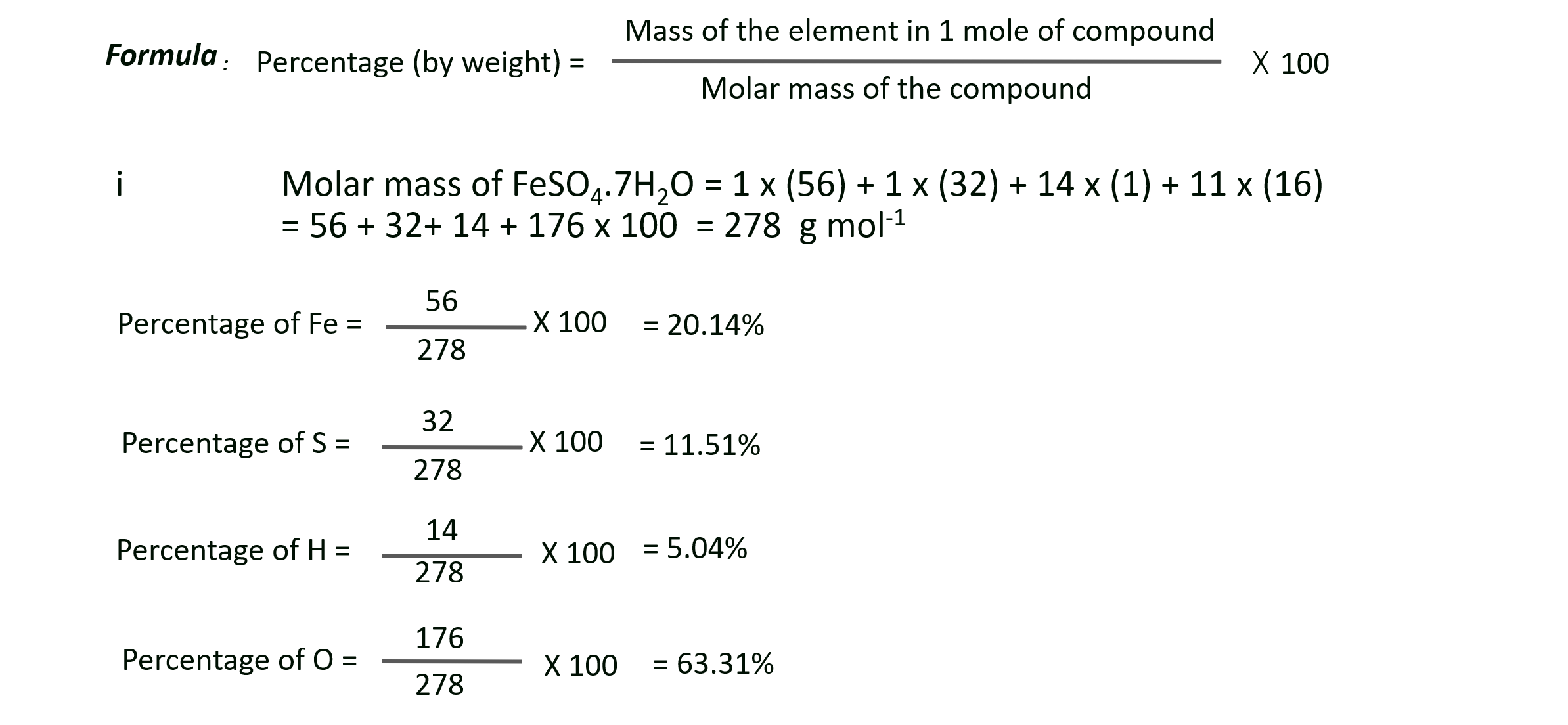

Ans: i. Mass percentage of Fe, S, H and O in FeSO4.7H2O are 20.14%, 11.51%, 5.04% and 63.31% respectively,
ii. Mass of iron in 4.54 kg green vitriol = 0.915 kg
Mass of water of crystallisation in 4.54 kg green vitriol = 2.058 kg
G. The red colour of blood is due to a compound called “haemoglobin”. It contains 0.335 % of iron. Four atoms of iron are present in one molecule of haemoglobin. What is its molecular weight ? (At. mass : Fe 55.84)
Solution:
Given : Iron percentage in haemoglobin = 0.335%
To Find : Molecular weight of haemoglobin
Calculation : There are four atoms of iron in a molecule of haemoglobin. Four atoms of iron contribute 0.335%
mass to a molecule of haemoglobin.
Mass of one Fe atom = 55.84 u
Mass of 4 Fe atoms = 55.84 x 4 = 223.36 u = 0.335%
Let molecular weight of haemoglobin be x.
Hence,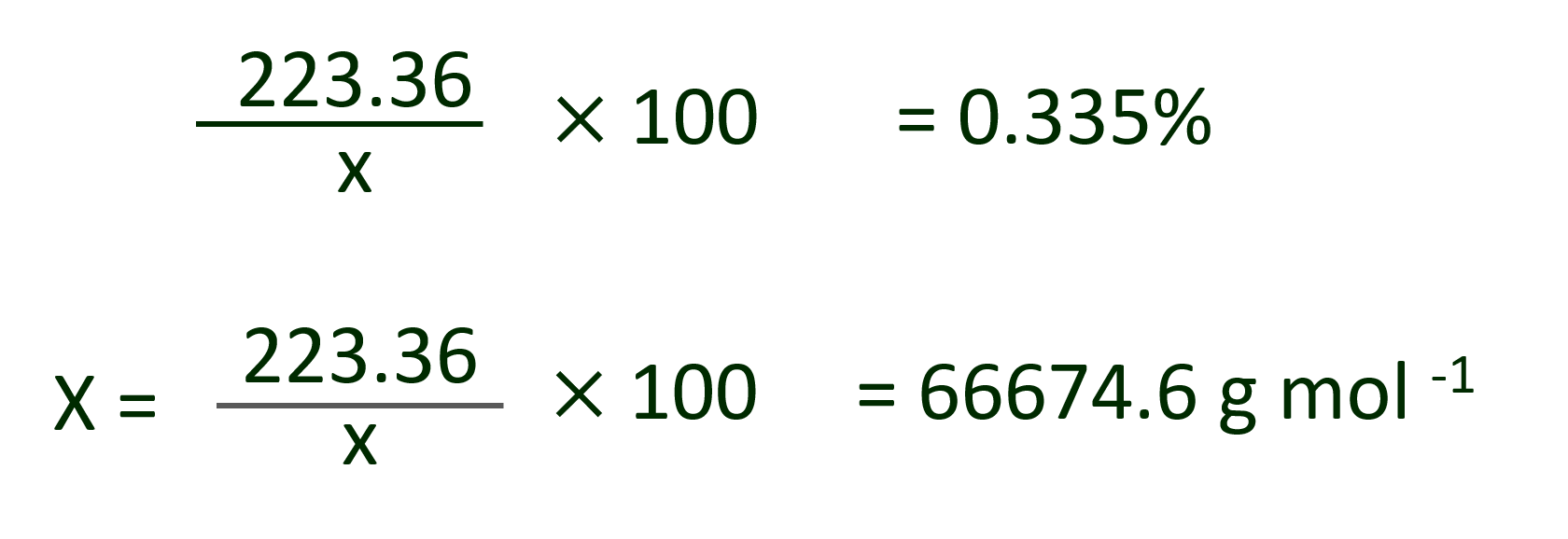
Ans : Molecular weight of haemoglobin = 66674.6 g mol–1
H. A substance, on analysis, gave the following percent composition:
Na = 43.4 %, C = 11.3 % and O = 45.3 %. Calculate the empirical formula. (At. mass Na = 23 u, C = 12 u, O = 16 u).
Solution : 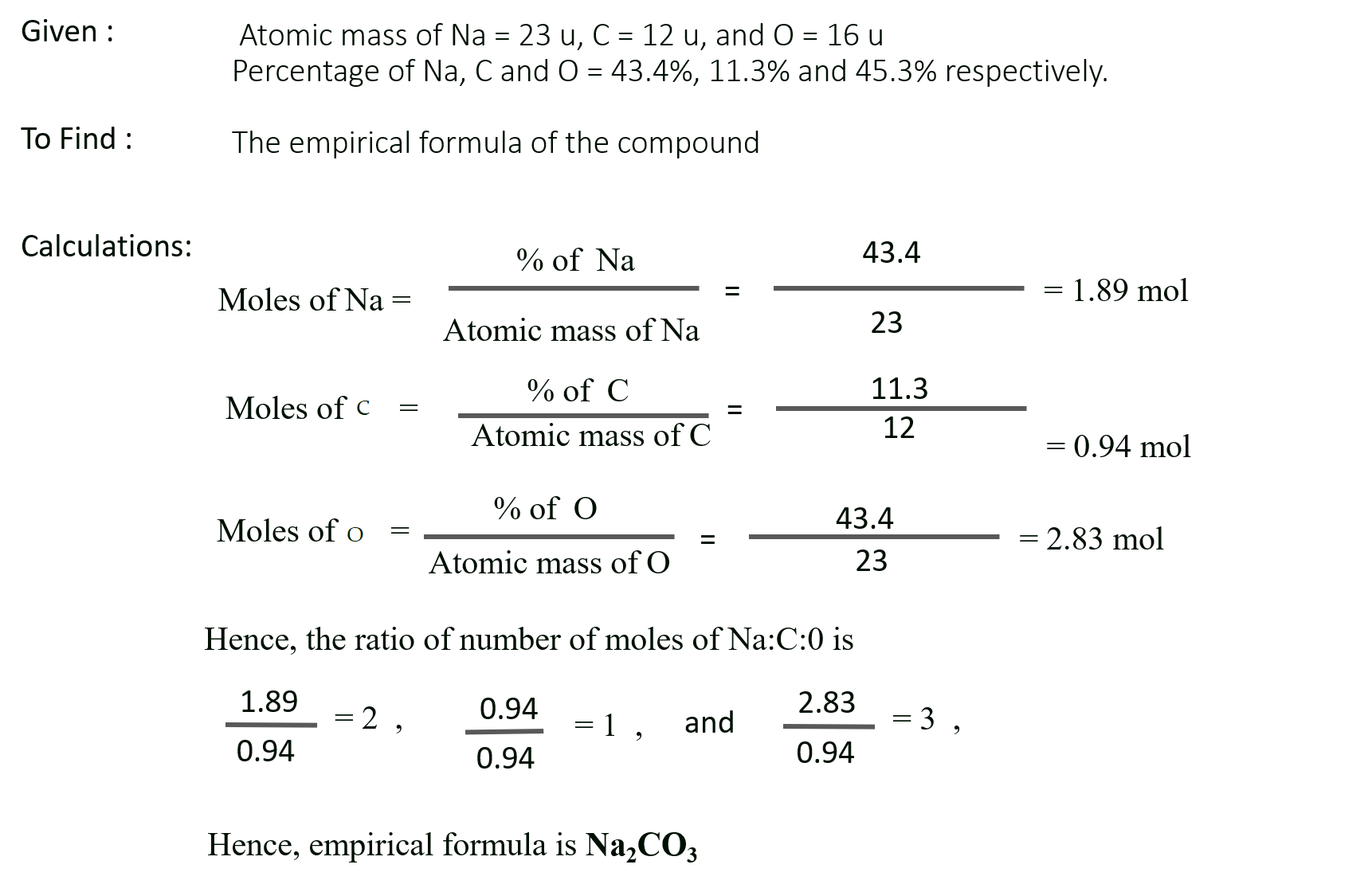
Ans: Empirical formula of the compound = Na2CO3
I. Assuming the atomic weight of a metal M to be 56, find the empirical formula of its oxide containing 70.0% of M.
Solution:
Given : Atomic mass of M = 56
Percentage of M = 70.0%
To Find : The empirical formula of the compound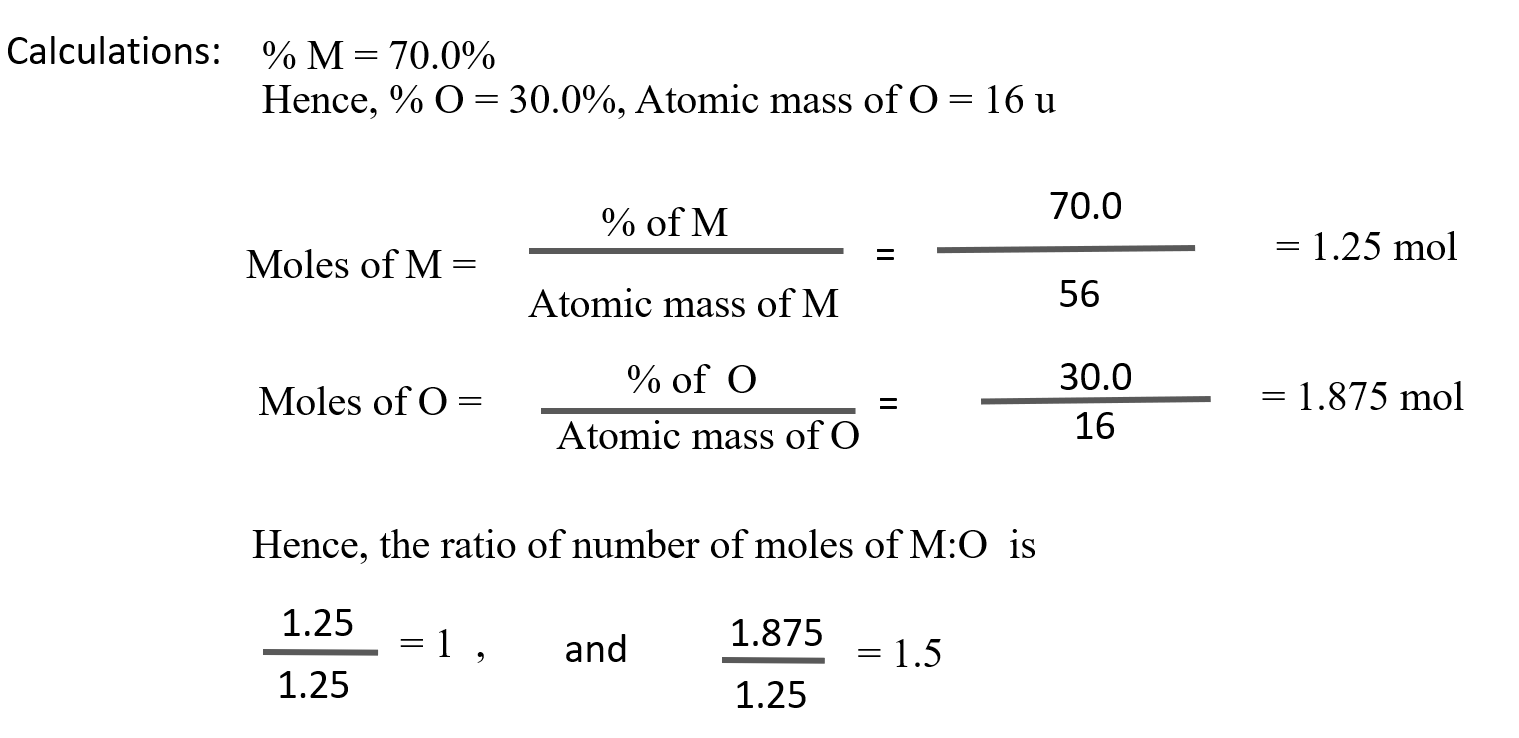
Convert the ratio into whole number by multiplying by the suitable coefficient, i.e., 2,
Therefore, the ratio of number of moles of M:O is 2:3.
Hence, the empirical formula is M2O2
Ans: Empirical formula of the compound = M2O3
J. 1.00 g of a hydrated salt contains 0.2014 g of iron, 0.1153 g of sulfur, 0.2301 g of oxygen and 0.4532 g of water of crystallisation. Find the empirical formula. (At. wt. : Fe = 56; S = 32; O = 16)
Solution:
Given : Atomic mass of Fe = 56, S = 32, and 0 = 1 6
Mass of iron, sulphur, oxygen and water = 0.2014 g, 0.1153 g, 0.2301 g and 0.4532 respectively.
To Find : The empirical formula of the compound
Calculation : Since the mass of crystal is 1 g, the % iron, sulphur, oxygen and water = 20.14%, 11.53%, 23.01% and 45.32% respectively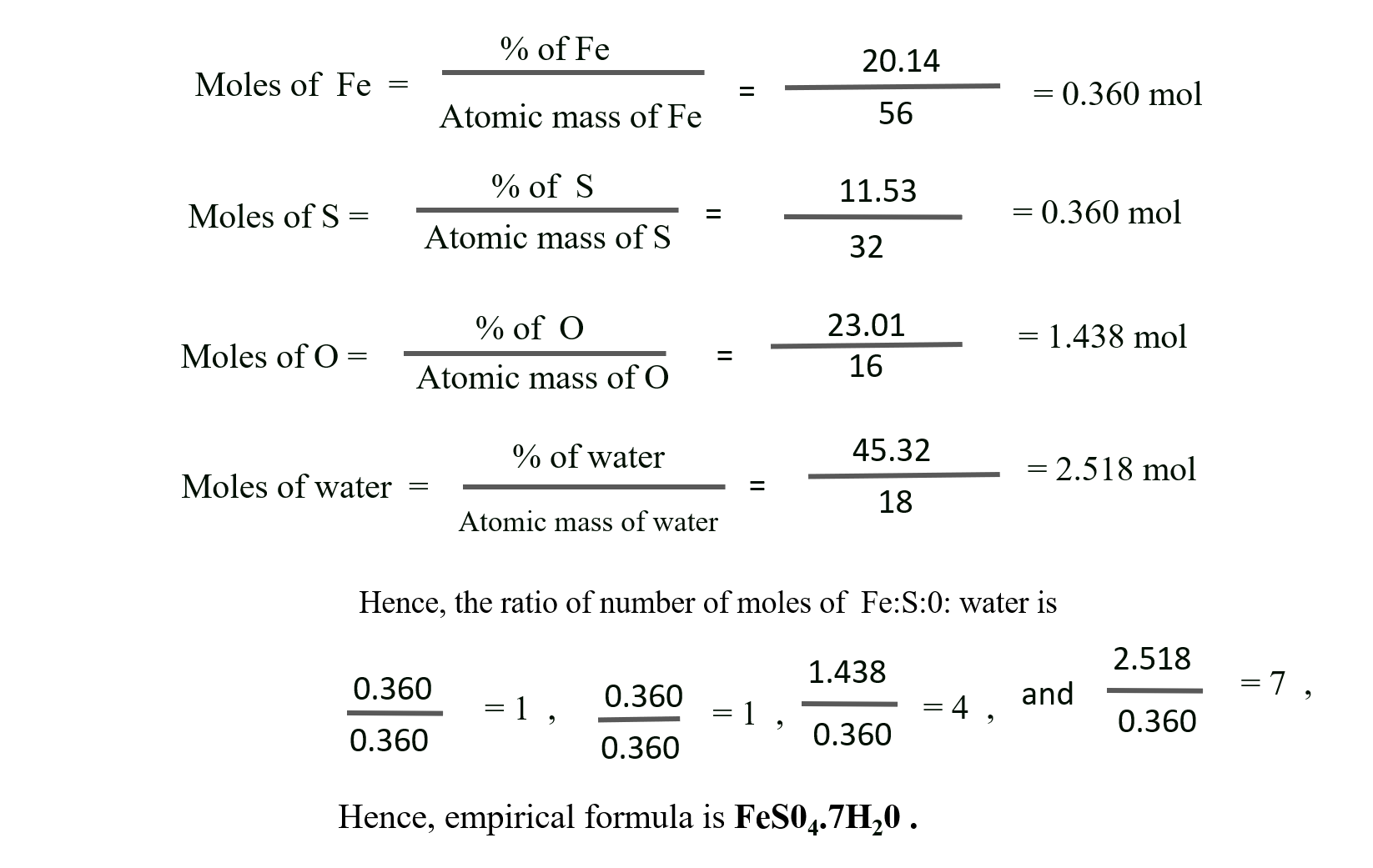
Ans: Empirical formula of the compound = FeS04.7H20.
K. An organic compound containing oxygen, carbon, hydrogen and nitrogen contains 20 % carbon, 6.7 % hydrogen and 46.67 % nitrogen. Its molecular mass was found to be 60. Find the molecular formula of the compound.
Solution:
Given : Percentage of carbon, hydrogen, nitrogen = 20%, 6.7%, 46.67% respectively.
Molar mass of the compound = 60 g mol–1
To Find : The molecular formula of the compound
Calculations: % carbon + % hydrogen + % nitrogen = 20 + 6.7 + 46.67 = 73.37%
This is less than 100%. Hence, compound contains adequate oxygen so that the total percentage of elements is 100%.
Hence, % of oxygen = 100 – 73.37 = 26.63%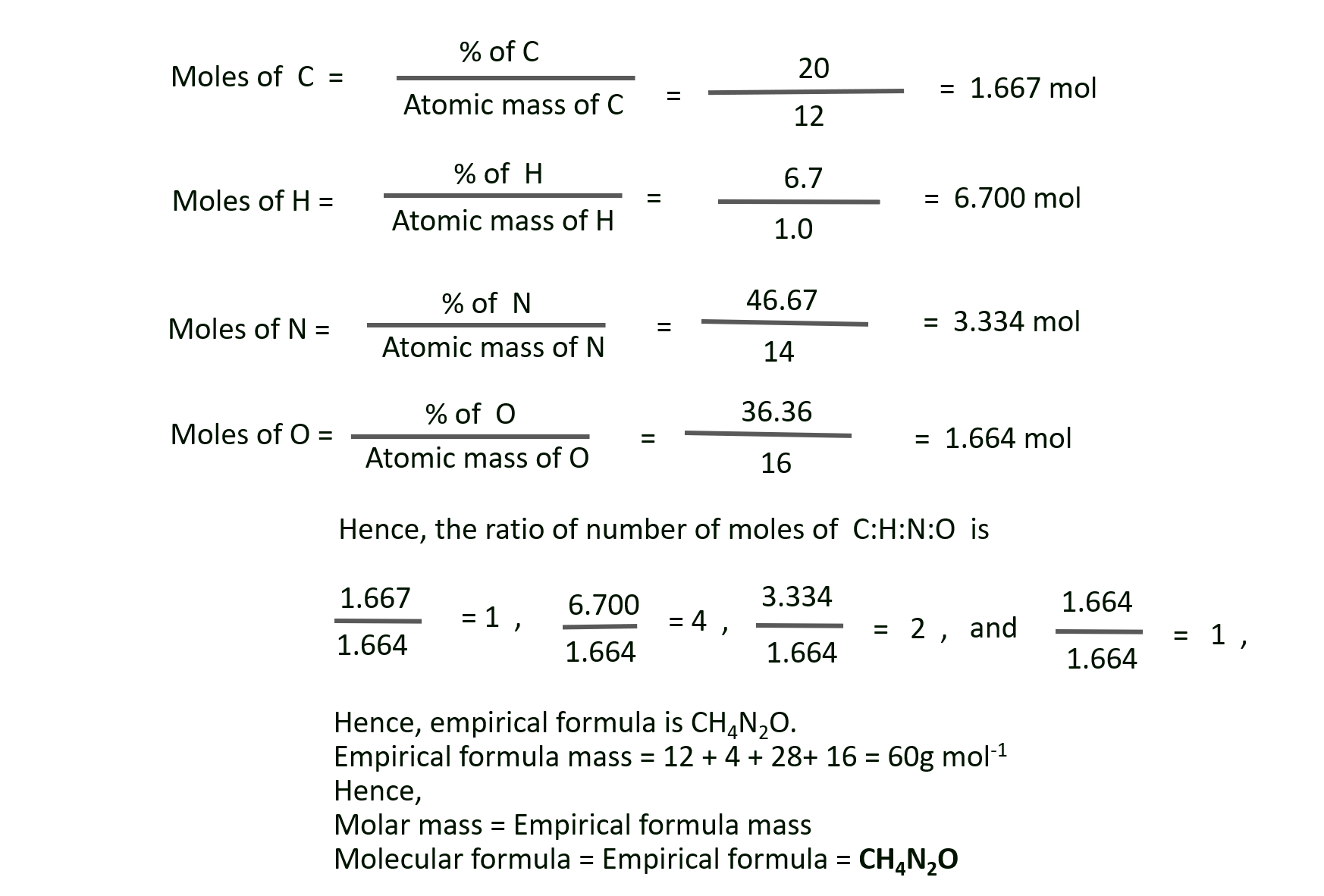
Ans: Molecular formula of the compound = CH2N2O
L. A compound on analysis gave the following percentage composition by mass : H = 9.09; O = 36.36; C = 54.55 Mol mass of compound is 88 g mol–1 Find its molecular formula.
Solution :
Given: Percentage of H, O, C = 9.09%, 36.36%, 54.55% respectively.
Molar mass of the compound = 88 g mol–1
To find: The molecular formula of the compound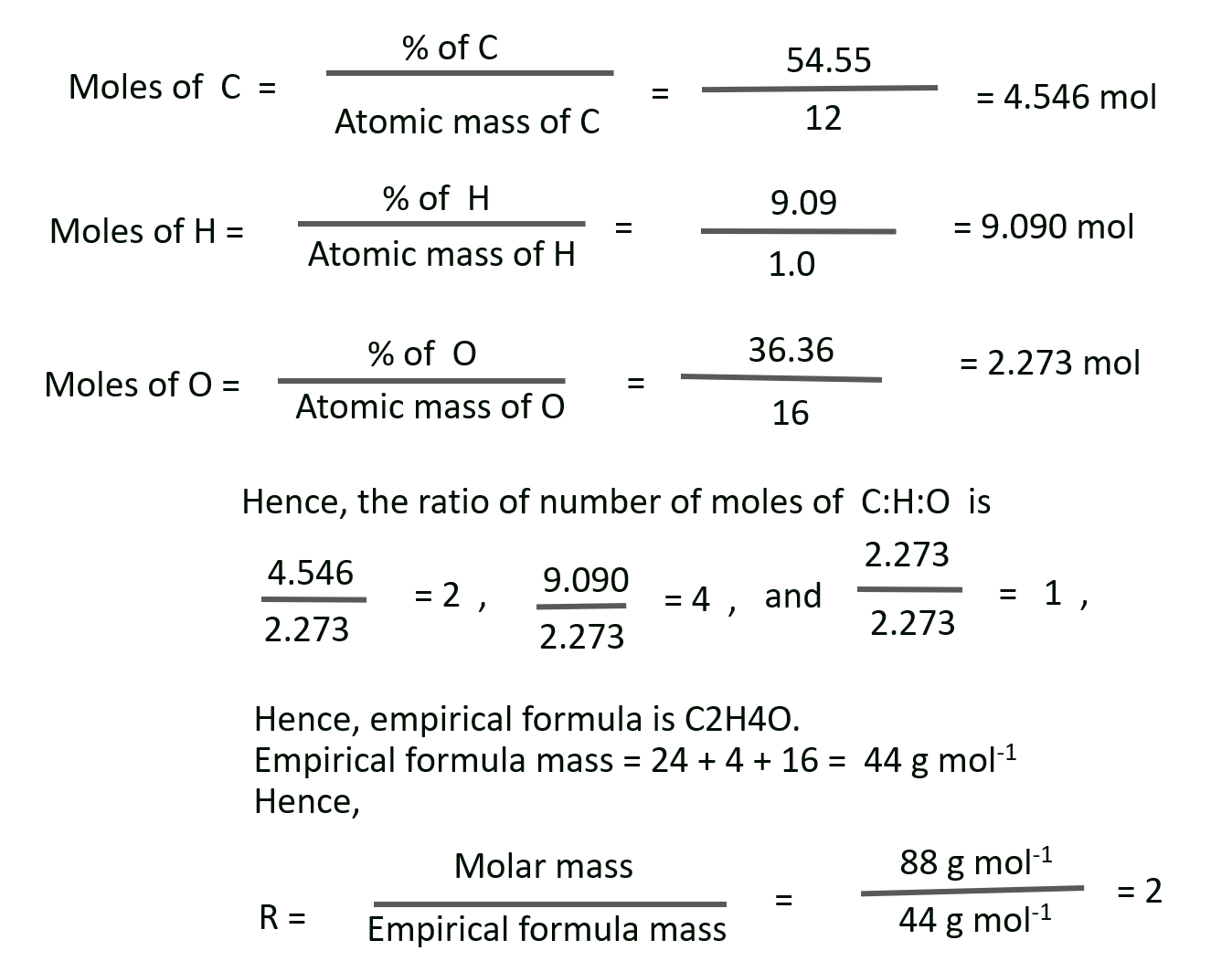
Molecular formula = r x empirical formula
Molecular formula = 2 x C2H4O = C4H8O2
Ans: Molecular formula of the compound = C4H8O2
M. Carbohydrates are compounds containing only carbon, hydrogen and oxygen. When heated in the absence of air, these compounds decompose to form carbon and water. If 310 g of a carbohydrate leave a residue of 124 g of carbon on heating in absence of air, what is the empirical formula of the carbohydrate ?
Solution :
Given : Mass of carbon residue = 124 g, mass of carbohydrate = 310 g
To Find : Empirical formula of the carbohydrate
Calculations : Since the 310 g of compound decomposes to carbon and water and the mass of carbon produced is 124 g, the remaining mass would be of water.
Mass of water = 3 1 0 – 124 = 186 g
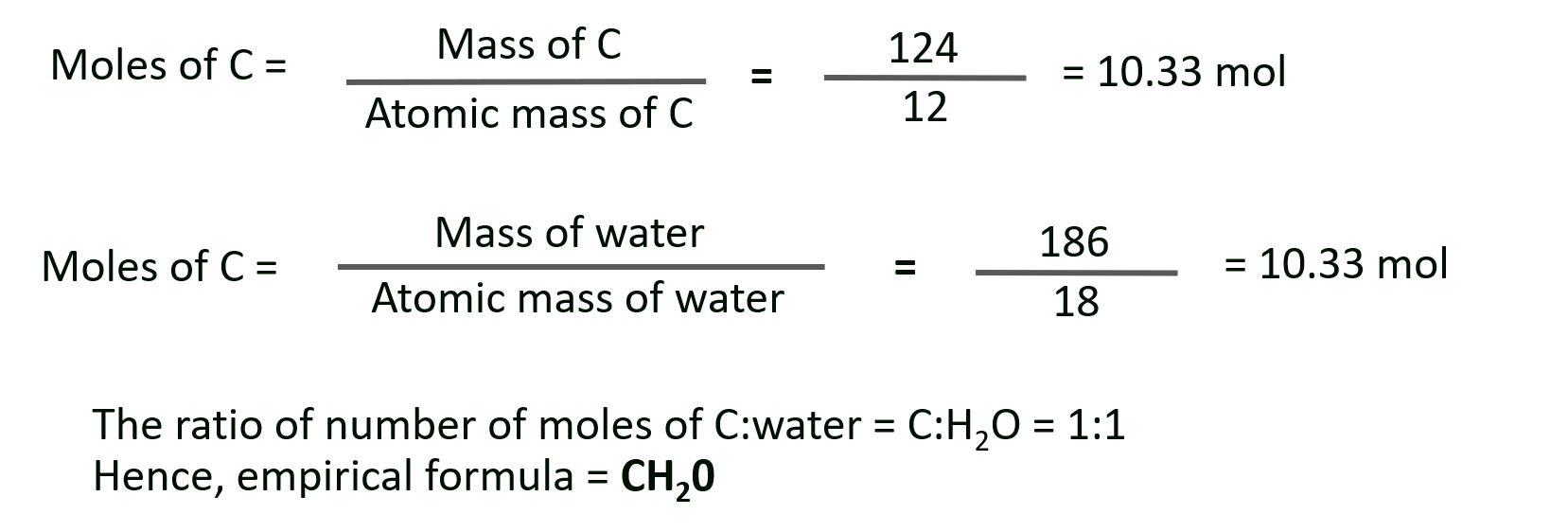
Ans: Empirical formula of the carbohydrate = CH2O
N. Write each of the following in exponential notation :
a. 3.672.199 b. 0.000098
c. 0.00461 d. 198.75
Ans:
i. 3,672,199 = 3.672199 x 106 ii.0.000098 = 9.8 x 10-5
iii. 0.00461 =4.61 x 10-3 iv.198.75 = 1.9875 x 10b2
O. Write each of the following numbers in ordinary decimal form :
a. 3.49 × 10–11 b. 3.75 × 10–1
c. 5.16 × 104 d. 43.71 × 10–4
e. 0.011 × 10–3 f. 14.3 × 10–2
g. 0.00477 × 105 h. 5.00858585
Ans :
a. 3.49 × 10–11 = 0.0000000000349
b. 3.75 × 10–1 = 0.375
c. 5.16 × 104 = 51.600
d. 43.71 × 10–4 = 0.004371
e. 0.011 × 10–3 = 0.000011
f. 14.3 × 10–2 = 0.143
g. 0.00477 × 105 = 477
h. 5.00858585 = 5.00858585
P. Perform each of the following calculations. Round off your answers to two digits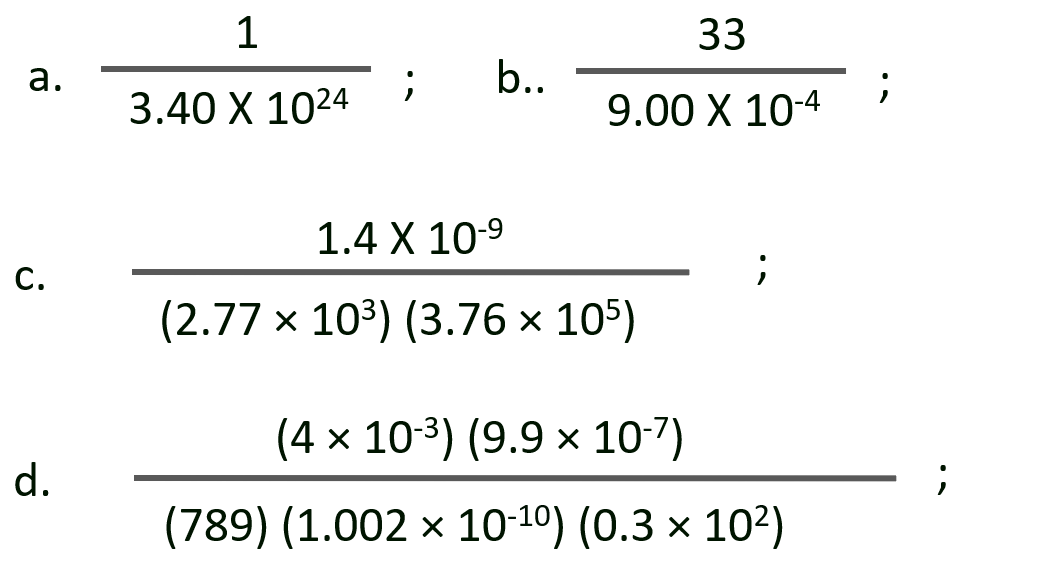
Solution : 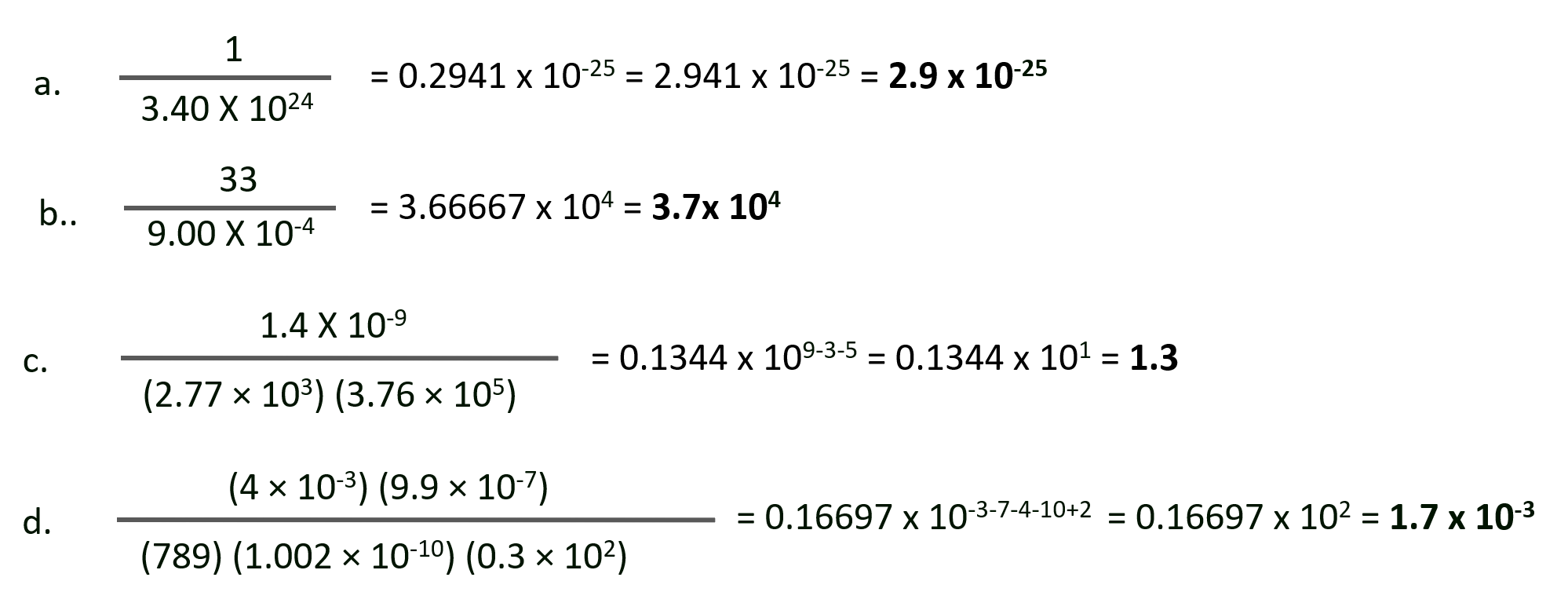
Q. Perform each of the following calculations. Round off your answers to three digits.
a. (3.26 104) (1.54 106)
b. (8.39 107) (4.53 109)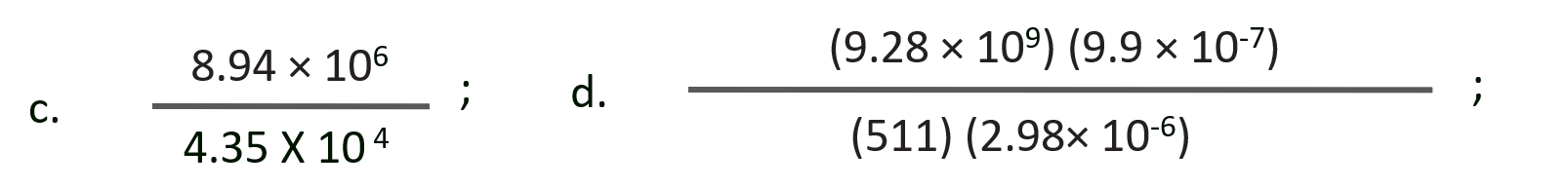
Solution :
a. (3.26 104) (1.54 106) = (3.26 x 1o4) x (1.54 x 10°) = 5.0204 x 104+6 = 5.02 x 1010
b. (8.39 107) (4.53 109) = (8.39 X 107) x (4.53 x 109) = 38.0067 x 107+9 = 38.0067 x 1016 = 3.80x 10117

R. Perform the following operations :
a. 3.971 × 107 + 1.98 × 104;
b. 1.05 × 10-5 – 9.7 × 10-5;
c. 4.11 × 10-3 + 8.1 10-4;
d. 2.12 × 106 – 3.5 × 10c.
Solution:
To perform addition/subtraction operation, first the numbers arc written in such a way that they have the
same exponent. The coefficients arc then added/subtracted.
i. 3.971 x 107 + 1.98 x 104 = 3.971 x 107 + 0.00198 x 107 = (3.971 +0.00198) x 107
= 3.97298 x 107
ii. 1.05 x 10-4– 9.7 x 10-5 = 10.5 x 10-5 – 9.7 x 1 O-5 = (10.5 – 9.7) x 10-5 = 0.80 x 10-5
= 8.0 x 1o-6
iii. 4.11 x 10-3 + 8.1 x 10-4 = 41.1 x 10-4 + 8.1 x 10-4 = (41.1 + 8.1)x 10-4 = 49.2 x 10-4
= 4.92 x 10-3
iv. 2.12 x 106 — 3.5 x 105 = 21.2 x 105 – 3.5 x 105 = (21.2 – 3.5) x 105= 17.7 x 105
= 1.77 x 1o6
S. A 1.000 mL sample of acetone, a common solvent used as a paint remover, was placed in a small bottle whose mass was known to be 38.0015 g. The following values were obtained when the acetone – filled bottle was weighed : 38.7798 g, 38.7795 g and 38.7801 g. How would you characterise the precision and accuracy of these measurements if the actual mass of the acetone was 0.7791 g ?
Solution: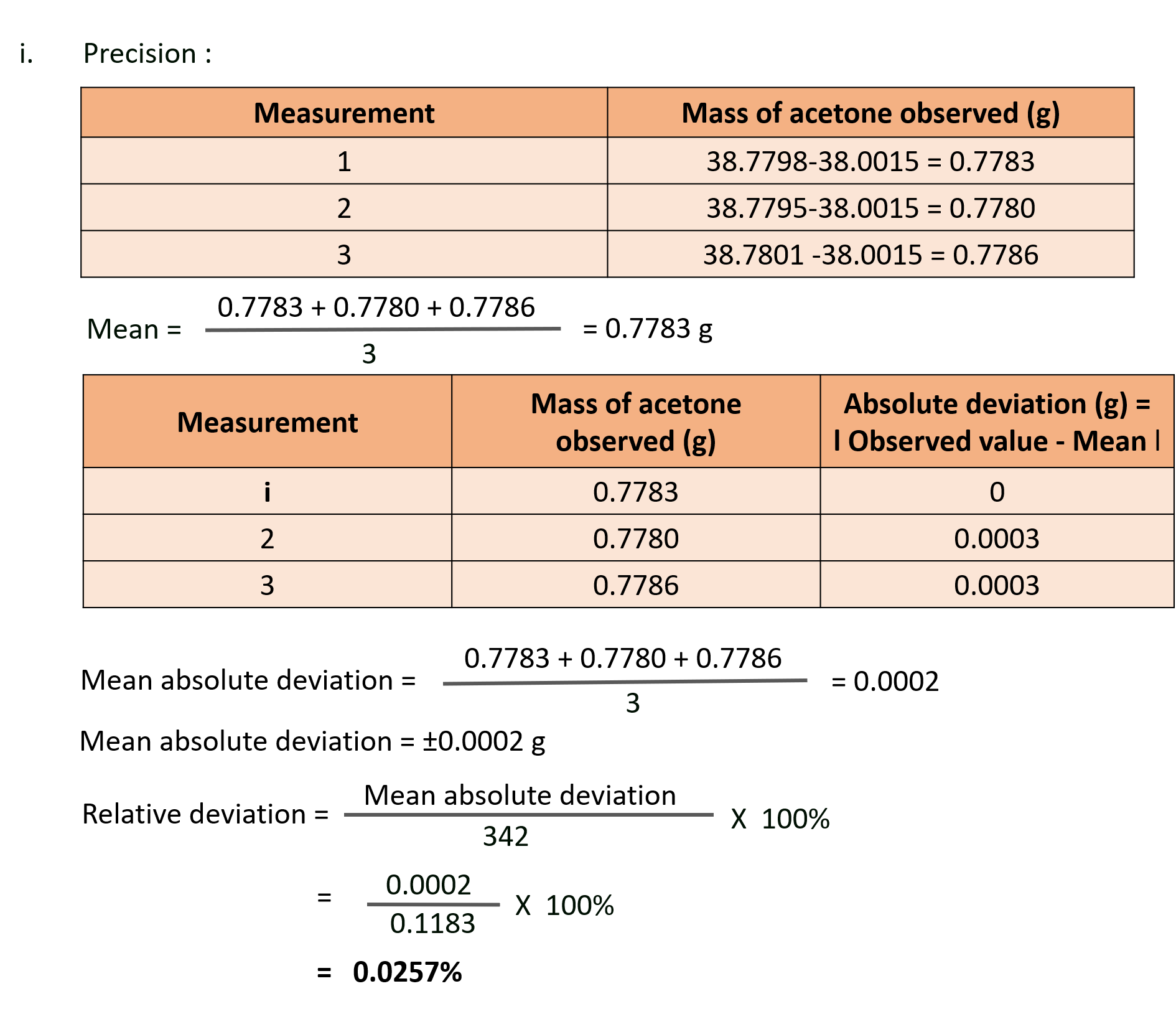
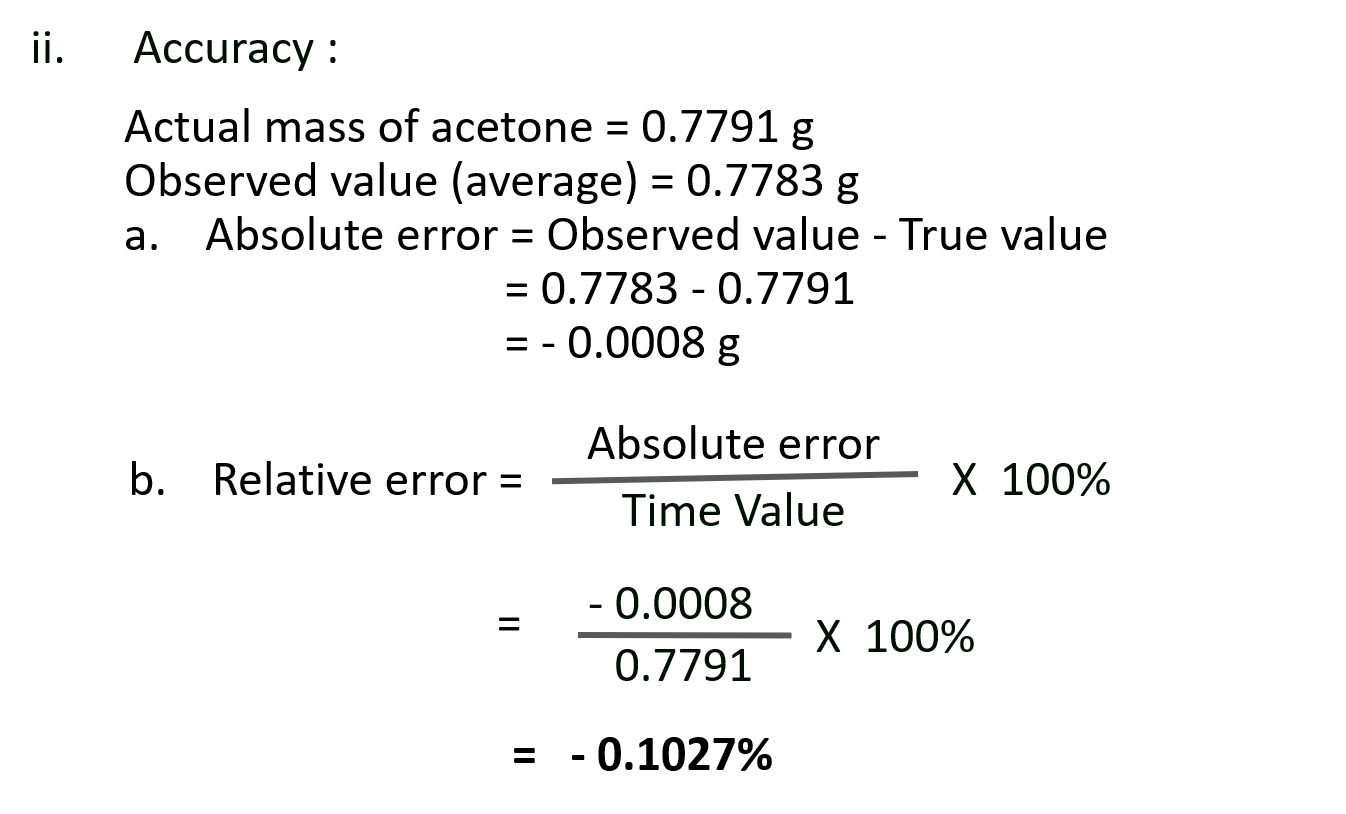
Ans: These observed values are close to each other and are also close to the actual mass. Therefore, the results are precise and as well accurate.
i. Relative deviation = 0.0257%
ii. Relative error = 0.1027%
T. Your laboratory partner was given the task of measuring the length of a box (approx 5 in) as accurately as possible, using a metre stick graduated in milimeters. He supplied you with the following measurements: 12.65 cm, 12.6 cm, 12.65 cm, 12.655 cm, 126.55 mm, 12 cm.
a. State which of the measurements you would accept, giving the reason.
b. Give your reason for rejecting each of the others
Ans :
a. The metre stick is graduated in millimetres i.e. 1 mm to 1000 mm, and 1 mm = 0.1 cm. Therefore, if length is
measured in centimetres, the least count of metre stick is 0.1 cm. The results 12.6 cm has the least count of
0.1 cm and is acceptable result.
b. Since, the least count of metre stick is 0.1 cm or 1mm, the results such as 12.65 cm, 12.655 cm, 126.55 mm
cannot be measured using this stick and hence, these results are rejected. The result, 12 cm doesn’t include
the least count and is rejected.
U. What weight of calcium oxide will be formed on heating 19.3 g of calcium carbonate ?
(At. wt. : Ca = 40; C = 12; O = 16)
Solution :
Given : Mass of CaC03 consumed in reaction = 19.3 g
To Find : Mass of CaO formed
Calculation : Calcium carbonate decomposes according to the balanced equation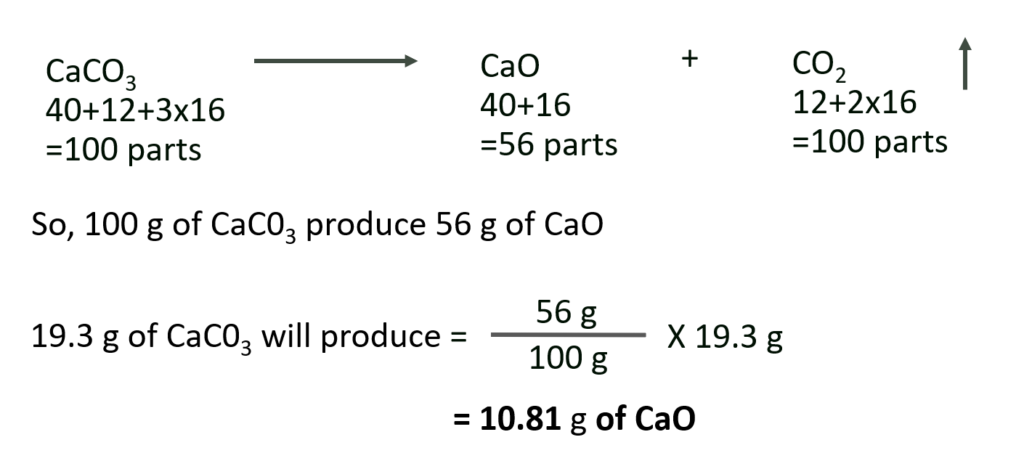
Ans: Mass of CaO formed = 10.81 g
V. The hourly energy requirements of an astronaut can be satisfied by the energy released when 34 grams of sucrose are “burnt” in his body. How many grams of oxygen would be needed to be carried in space capsule to meet his requirement for one day ?
Solution : 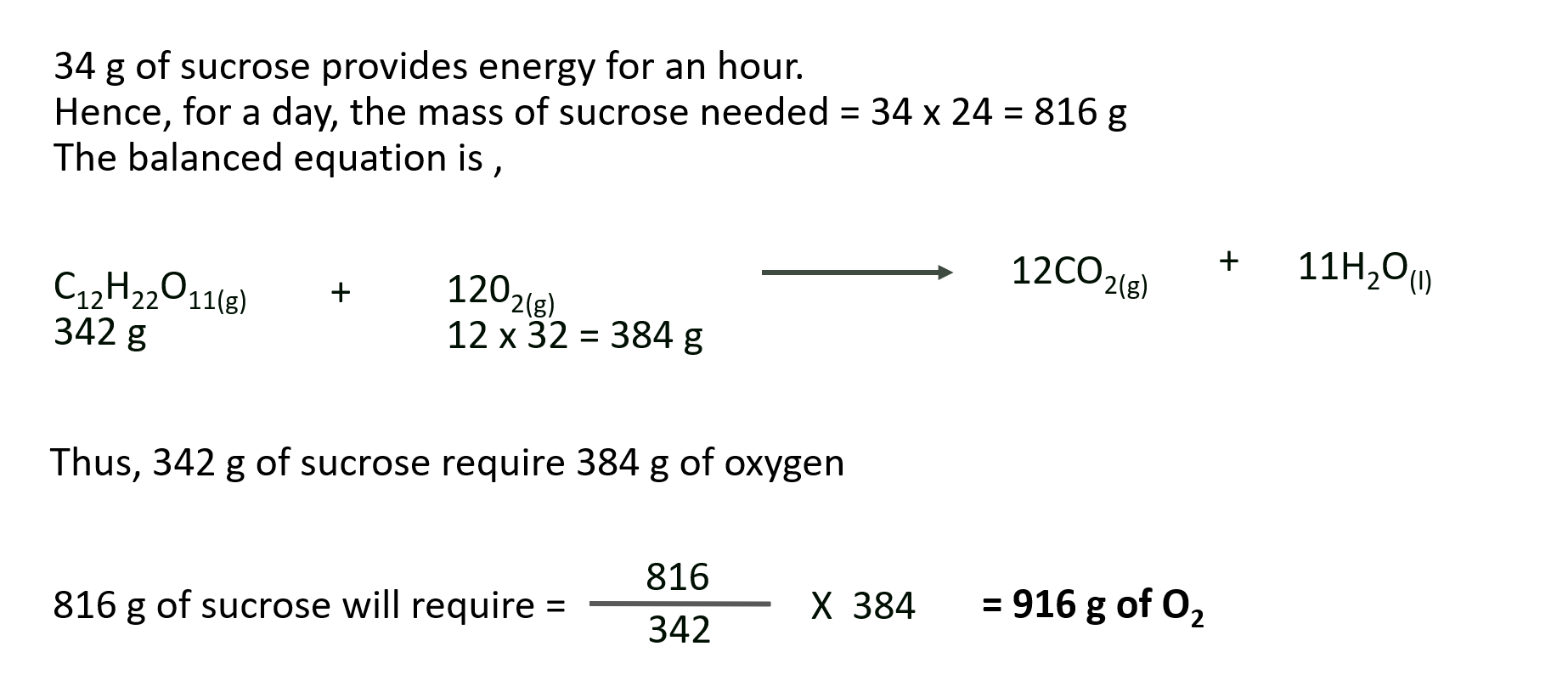
Ans: Astronaut needs to carry 916 g of O2.
