1. Choose the correct option from the bracket and explain the statement giving reason.
(Oxidation, displacement, electrolysis, reduction, zinc, copper, double displacement, decomposition)
a. To prevent rusting, a layer of zinc metal is applied on iron sheets.
b. The conversion of ferrous sulphate to ferric sulphate is oxidation reaction.
c. When electric current is passed through acidulated water decomposition/electrolysis of water takes place.
d. Addition of an aqueous solution of ZnSO4 to an aqueous solution of BaCl2 is an example of double displacement reaction.
2. Write answers to the following.
a. What is the reaction called when oxidation and reduction take place simultaneously? Explain with one example.
Ans.
When oxidation and reduction take place simultaneously in a given reaction it is termed as Redox reaction. During oxidation a reactant combines with oxygen or loses hydrogen and during reduction it gains hydrogen or loses oxygen.
Example of Redox reaction:
CuO + H2 → Cu + H20
In this reaction, CuO loses oxygen to form Cu. This means that reduction of CuO takes place. H2 molecule takes up oxygen to form H20. This means that oxidation of H2 takes place.
b. How can the rate of the chemical reaction, namely, decomposition of hydrogen peroxide be increased?
Ans.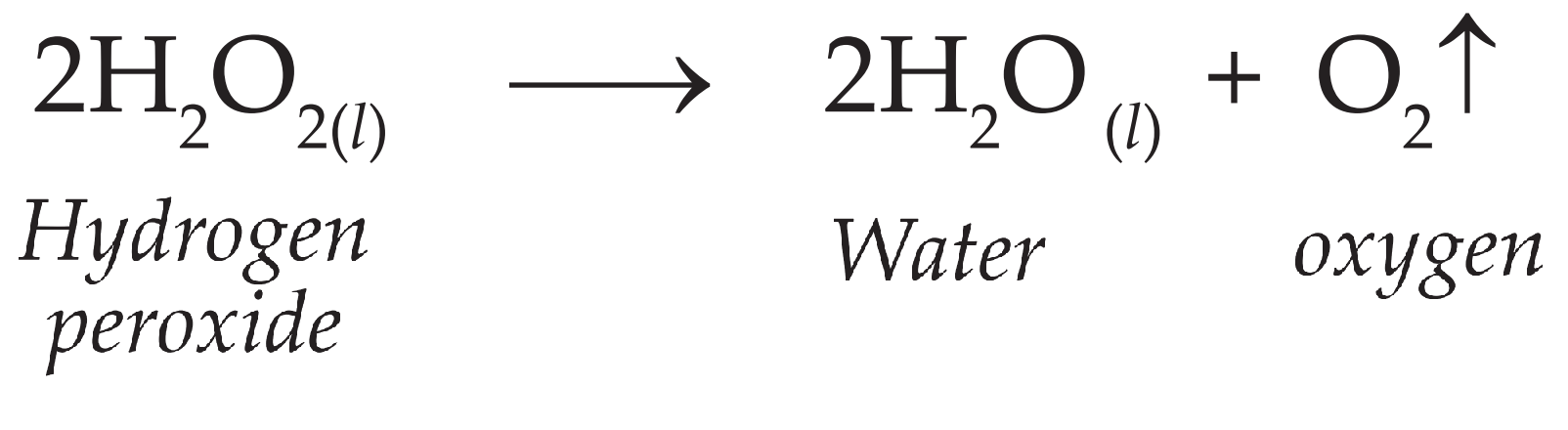
i. The decomposition of hydrogen peroxide (H2O2) into water and oxygen takes place slowly at room temperature.
ii. However, hydrogen peroxide decomposes rapidly in the presence of manganese dioxide (MnO2) powder, which acts as a catalyst.
iii. Thus, by adding manganese dioxide (MnO2) powder, the rate of chemical reaction can be increased.
c. Explain the term reactant and product giving examples.
Ans.
A chemical reaction is a process in which some substances undergo bond breaking and are transformed into new substances by formation of new bonds. The substances taking part in chemical reaction are called reactants, whereas the substances formed as a result of a chemical reaction by formation of new bonds are called products. For example
(i) Formation of carbon dioxide gas by combustion of coal in air is a chemical reaction. In this reaction Coal (carbon) and Oxygen (from air) are the reactants while carbon dioxide is the product.
C + O2 → CO2
Reactants product
d. Explain the types of reaction with reference to oxygen and hydrogen. Illustratre with examples.
Ans.
Types of reaction with reference to oxygen and hydrogen are oxidation reaction and reduction reaction.
Oxidation reaction: The chemical reaction in which a reactant combines with oxygen or loses hydrogen to form the product is called Oxidation equation.
Example:![]()
Here O2 combines with magnesium (Mg) to form magnesium oxide (MgO) 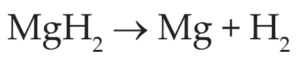
Here MgH2 loses hydrogen.
Reduction reaction: The chemical reaction in which a reactant combines with hydrogen or loses oxygen to form product is called reduction reaction.
Example: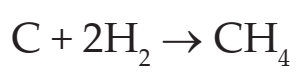
Here carbon combines with hydrogen and forms compound CH4 (Methane).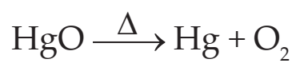
Here Mercuric oxide loses oxygen to form mercury.
e. Explain the similarity and difference in two events, namely adding NaOH to water and adding CaO to water.
Ans.
Similarity: Both NaOH and CaO dissolves in water. NaOH dissolves in water forming aqueous NaOH while CaO reacts with water to form Calcium Hydroxide [Ca(OH)2] Both are bitter in taste, and turns red litmus to blue. i.e. both are basic in nature.
Both are exothermic reaction.
After adding water, the properties of aqueous solution of sodium hydroxide (NaOH) and aqueous solution of calcium oxide CaO are different.
Difference: NaOH is highly soluble in water. Whereas calcium oxide CaO is less soluble in water. Aqueous solution of NaOH is monovalent. Whereas Aqueous solution of Ca(OH)2 is divalent. As NaOH is highly soluble in water, makes it strong base whereas Ca(OH)2 is a weak base.
3. Explain the following terms with examples.
a. Endothermic reaction
Ans:
(i) The chemical reactions which are accompanied by absorption of heat are called endothermic reactions.
(ii) Example: When calcium carbonate (CaCO3) is strongly heated, it undergoes decomposition to form calcium oxide (CaO) and carbon dioxide (CO2).

b. Combination reaction
Ans:
(i) When two or more reactants combine in a reaction to form a single product, it is called a combination reaction.
(ii) Example: When magnesium (Mg) strip is burnt in air, a white powder of magnesium oxide (MgO) is formed.
c. Balanced equation
Ans: A chemical equation in which the number of atoms of the elements in the reactants is same as the number of atoms of those elements in the products is called a balanced chemical equation.
![]()
d. Displacement reaction
Ans.
(i) The reaction in which the place of the ion of a less reactive element in a compound is taken by another more reactive element by formation of its own ions is called a displacement reaction.
(ii) Example: When zinc (Zn) dust is added to blue coloured copper sulphate (CuSO4) solution, a colourless solution of zinc sulphate (ZnSO4) is formed along with copper (Cu) metal.
4. Give scientific reasons.
a. When the gas formed on heating limestone is passed through freshly prepared lime water, the lime water turns milky.
Ans.
(i) On heating limestone, it undergoes thermal decomposition to form calcium oxide (quick lime) and carbon dioxide.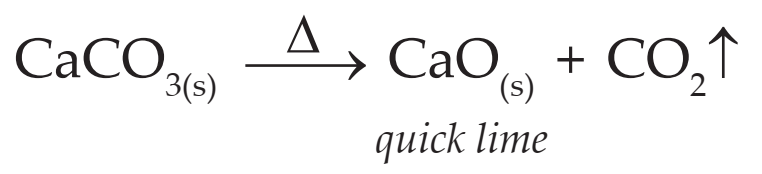
(ii) Calcium oxide dissolved in water forms calcium hydroxide (lime water).

(iii) When carbon dioxide is passed through lime water it turns milky due to formation of white precipitate of calcium carbonate.
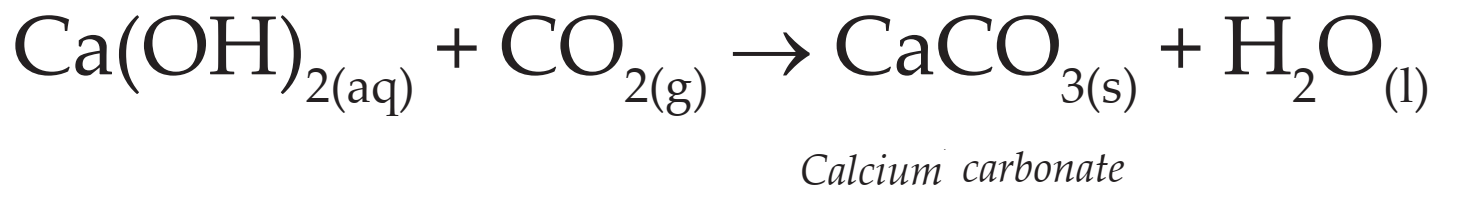
b. It takes time for pieces of Shahabad tile to disappear in HCl, but its powder disappears rapidly.
Ans.
(i) The rate of a chemical reaction depends upon the size of the reactant particles taking part in the reaction.
(ii) Smaller the size of the reactant particles, higher is the rate of reaction.
(iii) The size of reactant particles is more in pieces of Shahabad tile as compared to powder of Shahabad tile.
(iv) When HCl is added to pieces of Shahabad tile, the CO2 effervescence is formed slowly. However, when HCl is added to Shahabad powder, the CO2 effervescence is formed at a faster rate. Hence, it takes time for pieces of Shahabad tile to disappear in HCl, but its powder disappears rapidly.
c. While preparing dilute sulphuric acid from concentrated sulphuric acid in the laboratory, the concentrated sulphuric acid is added slowly to water with constant stirring.
Ans.
(i) In the process of dilution of concentrated sulphuric acid with water, very large amount of heat is liberated. This process is highly exothermic.
(ii) As a result, if water is added to concentrated sulphuric acid, then water gets evaporated instantaneously. The mixture may splash out causing an accident.
(iii) In order to prevent this, the required amount of water is taken in a glass container and small quantity of concentrated sulphuric acid at a time is added with constant stirring. So, only a small amount of heat is liberated at a time. Hence, while preparing dilute sulphuric acid from concentrated sulphuric acid in the laboratory, the concentrated sulphuric acid is added slowly to water with constant stirring.
d. It is recommended to use air tight container for storing oil for long time.
Ans.
(i) When edible oil is left aside for long time, it undergoes air oxidation.
(ii) Due to this, the taste and smell of oil changes and it becomes rancid. If food is cooked in this oil, its taste also changes.
(iii) Thus, the oil will become unfit for consumption.
(iv) The process of oxidation reaction of oil can be slowed down by storing it in air tight container. Hence, it is recommended to use air tight container for storing oil for
long time.
5. Observe the following picture a write down the chemical reaction with explanation.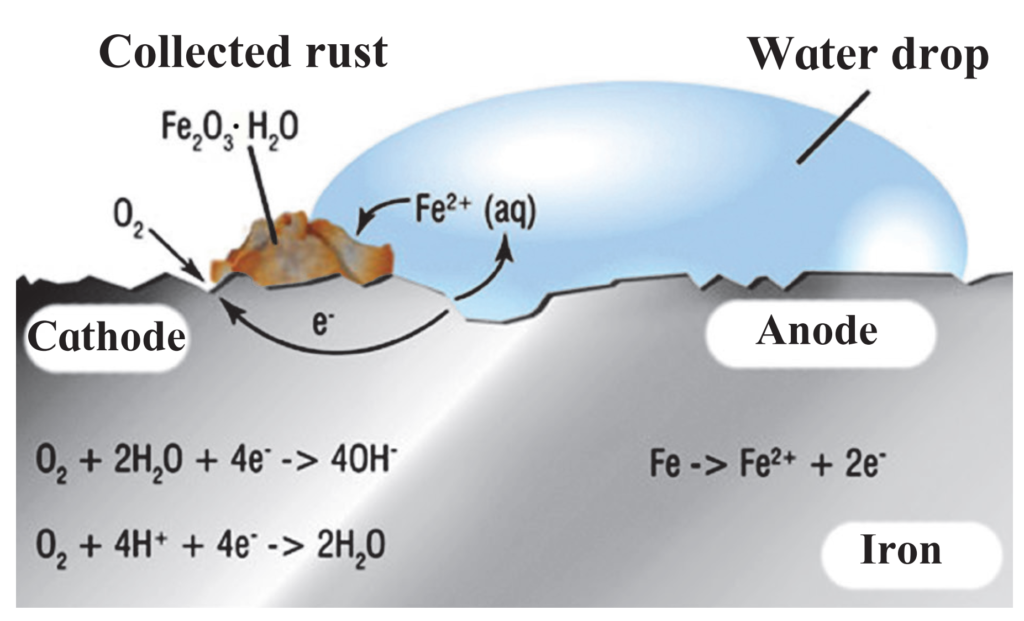
Ans:
(1) The given picture represents the electrochemical reaction taking place during the corrosion (rusting) of iron.
(2) Different regions on the surface of iron become anode and cathode.
(3) In the anode region, Fe is oxidized to Fe2+ .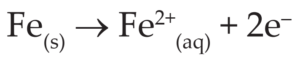
(4) The electron released in the anode region flow through the metal surface to the cathode region where they reduce oxygen.
In the cathode region, O2 is reduced to form water.
(5) When Fe2+ ions migrate from the anode region, they react with water (or OH– ions) and further get oxidized to Fe3+ ions.
(6) Fe3+ ions form an insoluble hydrated oxide (Fe2O3.H2O), which is deposited as reddish brown layer on the surface. It is called rust.
6. Identify from the following reactions the reactants that undergo oxidation and reduction.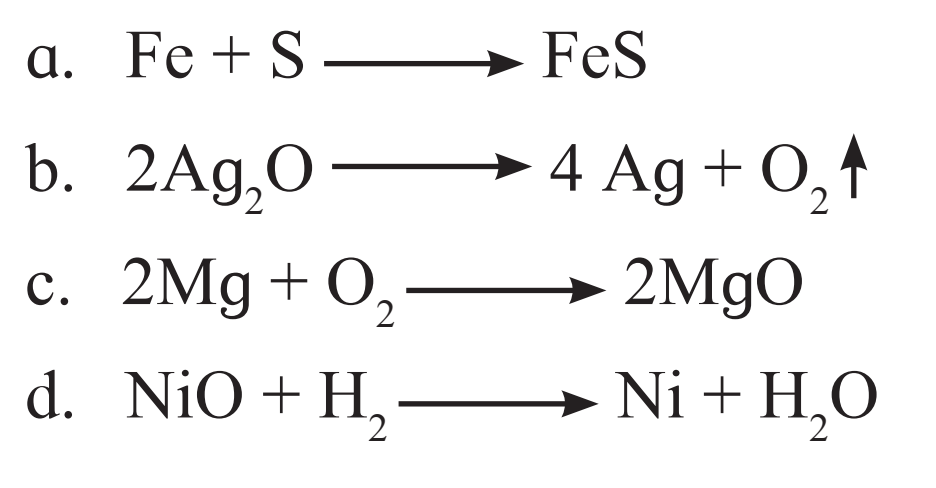
Ans.
a. Fe undergoes oxidation and S undergoes reduction.
b. Ag2O undergoes reduction.
c. Mg undergoes oxidation and O2 undergoes reduction.
d. NiO undergoes reduction and H2 undergoes oxidation.
7. Balance the following equation stepwise.
Ans.
8. Identify the endothermic and exothermic reaction.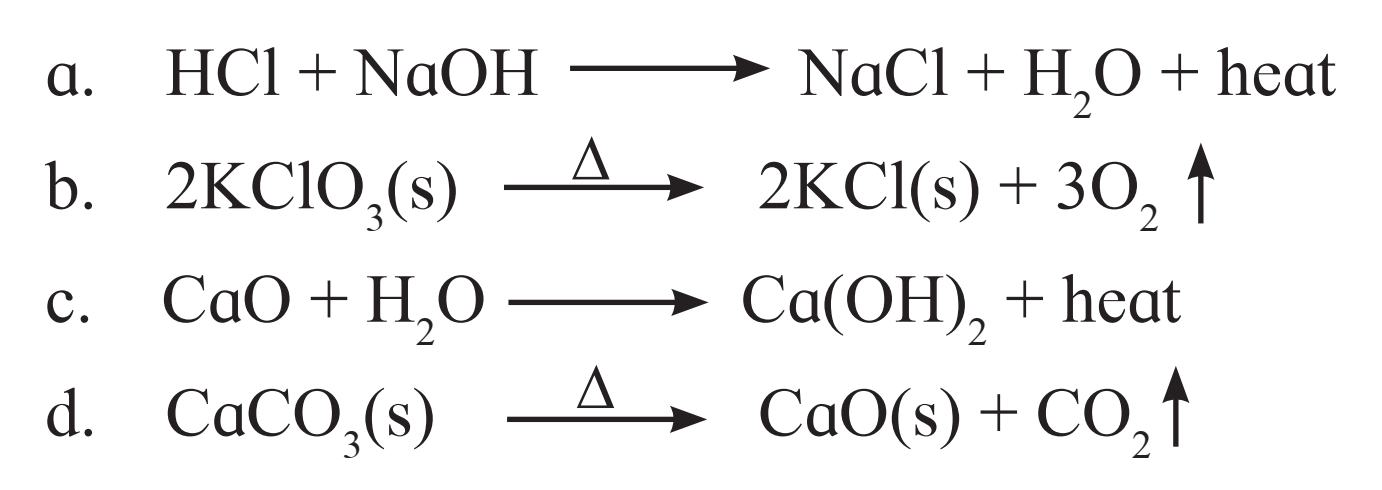
Ans.
a. Exothermic reaction
b. Endothermic reaction
c. Exothermic reaction
d. Endothermic reaction
9. Match the column in the following table.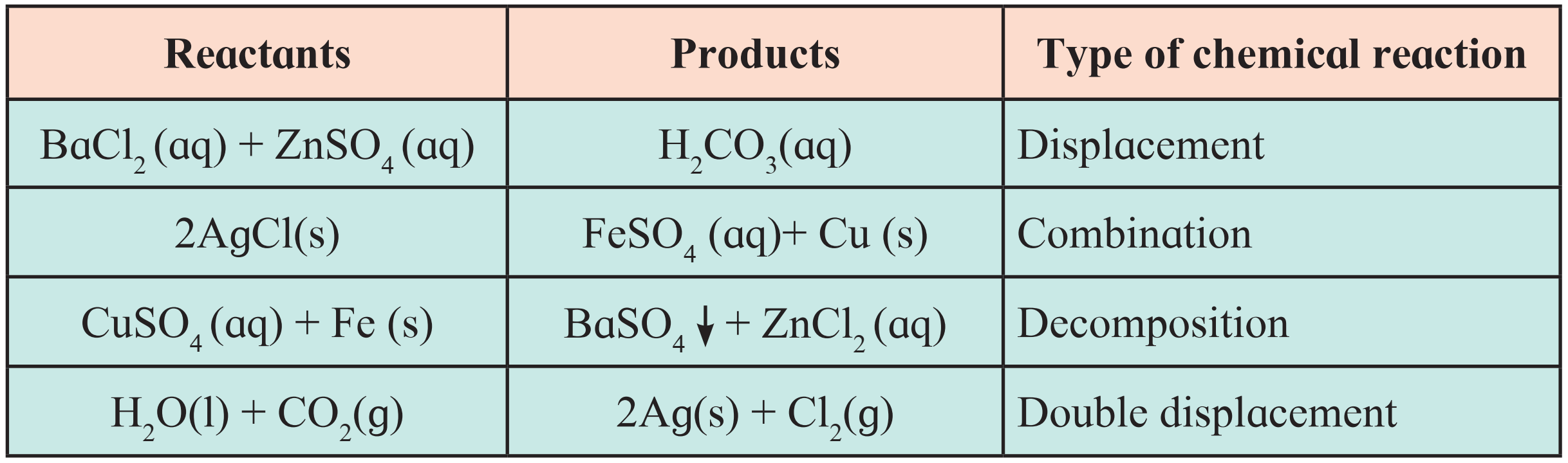
Ans.