Que 2 B) V. Explain concept with example / explain with the help of balanced equation. (Each 2 m)
1. Ionic bond and ionic compounds
Ans.
(1) The compounds formed from two units, namely cation and anion are called ionic compounds.
(2) The cation and anion being oppositely charged, there is an electrostatic force of attraction between them. This force of attraction between cation and anion is called as the ionic bond.
(3) The number of cations and anions in a compound and the magnitude of the electric charge on them is such that the positive and negative charges balance each other. As a result, an ionic compound is electrically neutral.
(4) For example, ionic compound sodium chloride (NaCl) is formed as sodium metal gives away one electron while the nonmetal chlorine takes up one electron. Na+ and Cl– ions formed are held together by attractive force called ionic bond.
2. Gangue
Ans.
(1) The unwanted impurities of sand, soil, rocky substances, etc., present in the ore are called gangue.
(2) Ores are taken out from the mines and the gangue is usually separated from the ore at the site itself by various methods.
(3) The process of separating gangue from the ores is called concentration of ores.
(4) For example, bauxite ore contains impurities of silica (SiO2), ferric oxide (Fe2O3) and titanium oxide (TiO2). These impurities are called gangue.
3. Ores
Ans.
(1) There can be many minerals from which a metal can be extracted.
(2) However, only those minerals from which a metal can be extracted profitably are called ores.
(3) Metals can be extracted from their ores by means of various methods of separation.
(4) For example, aluminium can be extracted profitably from its bauxite ore. Hence, bauxite is an ore of aluminium.
4. Roasting and calcination
Ans.
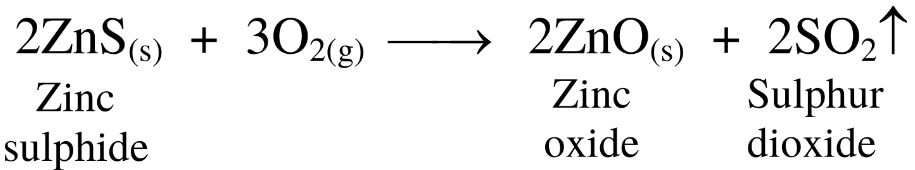
(1) The process of heating of sulphide ores strongly in excess of air to convert it into respective oxide is called roasting.
(2) For example, zinc sulphide is heated strongly in excess of air to convert it to zinc oxide.
(3) The process of heating carbonate ores in limited air to convert it into respective oxide is called calcination.
(4) For example, zinc carbonate is heated strongly in limited supply of air to convert it to zinc oxide.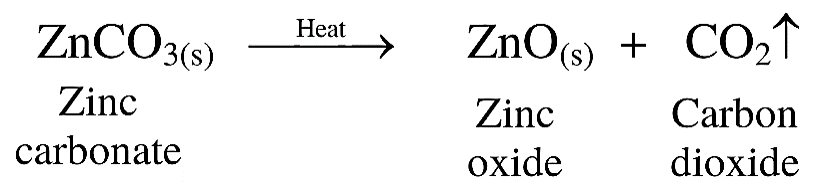
5. Corrosion
Ans.
(1) The process of slow degradation of the metals due to the attack of atmospheric gases, moisture, acids, etc. on the surface of metals is called corrosion.
(2) Iron reacts with moist air and a deposit of reddish substance (Fe2O3.H2O) is formed on it. This substance is called rust and the corrosion of iron is known as rusting.
(3) Carbon dioxide in moist air reacts with the surface of copper vessel. Copper loses its lustre due to formation of greenish layer of copper carbonate (CuCO3) on its surface. This is called patination of copper.
(4) Various methods such as galvanizing, tinning, alloying, etc. are used to protect metals from corrosion.
6. Minerals
Ans.
(1) Most metals being reactive do not occur in nature in free state but are found in combined state as their salts such as oxides, carbonates, sulphides and nitrates.
(2) However, some unreactive metals like silver, gold, platinum, generally occur in free state.
(3) The compounds of metals that occur in nature along with the impurities are called minerals.
(4) For example, bauxite is a mineral of aluminium.