1. A …………… is necessary to change the speed as well as the direction of motion of an object.
(A) force (B) inertia (C) momentum (D) motion
2. The orbit of a planet revolving around a star is …………..
(A) circular (B) linear (C) towards the focal point (D) elliptical
3. The square of its period of revolution around the sun is directly proportional to the ……………… of the mean distance of a planet from the sun.
(A) square (B) square root (C) cube (D) cube root
4. The gravitational force between two bodies is directly proportional to the product of the masses of those bodies and is …………………. of the distance between them.
(A) inversely proportional to the square (B) directly proportional to the square (C) inversely proportional to the cube (D) inversely proportional to the square root
5. The value of universal gravitational constant (G) in SI unit is ……………..
(A) 6.673 x 10-11 Nm2/ kg2 (B) 6.673 x 1011 Nm/ kg (C) 6.673 x 10-11 Nm/ kg (D) 9.673 x 10-11 Nm2/ kg2
6. The …………….. force is much weaker than other forces in nature.
(A) gravitational (B) electromagnetic (C) nuclear force (D) intermolecular
7. The value of gravitational acceleration (g) is …………..
(A) highest at the poles (B) highest at the equator (C) same everywhere on the surface of the earth (D) lowest at the poles
8. The value of gravitational acceleration (g) is …………….. at the equator.
(A) 9.78 m/s2 (B) 9.832 m/s2 (C) 9.8 m/s2 (D) 6.67 m/s2
9. The free fall of an object is possible only in ……………..
(A) air (B) vacuum (C) on the surface of earth (D) None of these
10. The weight of any object on the moon is nearly ……………. of the weight of the earth.
(A) 1/6 (B) 1/8 (C) 1/2 (D) 2/6
11. A person weighs 60 N on earth. His weight on the moon will be ………….
(A) 360 N (B) 60 N (C) 6 N (D) 10N
12. Dobereiner laid down the …………… rule.
(A) periodic (B) modem periodic (C) triad (D) octaves
13. Newlands’ Law of Octaves is applicable upto ……………
(A) oxygen (B) calcium (C) cobalt (D) potassium
14. X and Y are two elements having similar properties which obey Newlands’s Law of Octaves. The minimum and maximum number of elements in between X and Y respectively are …………..
(A) 6 and 8 (B) 7 and 15 (C) 8 and 14 (D) 6 and 13
15. At the time of Mendeleev ……………. elements were known.
(A) 56 (B) 65 (C) 63 (D) 118
16. In Mendeleev’s periodic table eka- silicon was later named as ……………..
(A) scandium (B) gallium (C) germanium (D) thorium
17. In Modem Periodic Table the number of columns and periods are respectively ……………. and …………….
(A) 16,7 (B) 6,16 (C) 18,7 (D) 18,6
18. ……………. is the outermost shell for elements of period 2.
(A) K (B) L (C) M (D) N
19. The groups 1 and 2 constitute the ……………. block.
(A) s (B) p (C) d (D) d
20. Which pair of atomic numbers represents elements in the same group?
(A) 11,19 (B) 6,12 (C) 4, 16 (D) 8, 17
21. Which among the following elements would lose an electron easily?
(A) Mg (B) Na (C) A1 (D) Cl
22 Which among the following is the largest element?
(A) Na (B) Mg (C) K (D) Ca
23. Arrange the following elements in order of their decreasing metallic character.
Na, Si, Cl, Mg, Al
(A) Cl > Si > Al > Mg > Na
(B) Na > Mg > Al > Si > Cl
(C) Na > Al > Mg > Cl > Si
(D) Al > Na > Si > Ca > Mg
24. Which one of the following does not increase while moving down the group of the Modern periodic table?
(A) Atomic radius (B) Metallic character (C) Valency (D) Number of shells
25. On moving from left to right in a periodic table, the size of the atom …………….
(A) increases (B) decreases (C) decreases first and then increases (D) does not change
26. Which of the following statements about the Modem periodic table is correct?
(A) 18 horizontal rows are known as Periods.
(B) 7 vertical columns are known as Periods.
(C) 18 vertical columns are known as groups.
(D) 7 horizontal rows are known as groups.
27. The d-block elements arc called as ………….. elements.
(A) transition (B) metalloid (C) normal (D) inner transition
28. The size of an atom is indicated by its …………..
(A) atomic number (B) radius (C) number of shells (D) atomic mass
29. …………… is the distance between the nucleus of the atom and its outermost shell.
(A) Atomic radius (B) Atomic diameter (C) atomic mass (D) atomic size
30. Atomic radius is expressed in the unit ………….
(A) nanometer (B) picometer (C) micrometer (D) millimeter
31. The tendency of an element to form cation is the …………….. character of that element.
(A) non metallic (B) basic (C) metallic (D) acidic
32. ……………….. is in liquid form in the halogen family.
(A) Fluorine (B) Chlorine (C) Bromine (D) Iodine
33. While going from top to bottom in a group the atomic radius ……………
(A) increases (B) decreases (C) remains same (D) No change occurs
34. The tendency of an element to form anion is the ……………. character of that element.
(A) nonmetallic (B) basic (C) metallic (D) acidic
35. The elements from the zero group are called ………….
(A) alkali metals (B) alkaline earth metals (C) halogen (D) noble gases
36. Writing a chemical reaction in brief by using chemical formulae is called as ……………..
(A) chemical change (B) chemical symbol (C) chemical equation (D) chemical reaction
37. When the positive charge on an ion increases or the negative charge on them decreases it is called as ……………..
(A) reduction (B) corrosion (C) oxidation (D) decomposition
38. The chemical reaction in which two or more products are formed from a single reactant is called ……………… reaction.
(A) decomposition (B) combination (C) displacement (D) double displacement
39. In the chemical equation the ……………. are written on the left hand side.
(A) products (B) reactants (C) element (D) catalyst
40. Aqueous solution of ZnS04 is added into the aqueous solution of BaCl2, this is the example of …………….. reaction.
(A) displacement (B) double displacement (C) redox (D) reduction.
41. The unit of electrical power is ………….
(A) volt (B) watt (C) joule (D) ampere
42. The ‘live’ and the ‘neutral’ wires have potential difference of ……………….
(A) 110 V (B) 202 V (C) 201V (D) 220 V
43. In an electric bulb, coil of …………….. metal is used.
(A) copper (B) tungsten (C) aluminium (D) iron
44. The electricity bill specifics the usage in ……………
(A) kilowatt (B) joule (C) volt (D) unit
45. The frequency of AC is ………….
(A) 20 Hz (B) 50 Hz (C) 25 Hz (D) 75 Hz
46. These days when current in the circuit suddenly increases …………… switches are used.
(A) MCA (B) MCC (C) MCD (D) MCB
47. A coil of an alloy …………….. is used in electric heater cooker as a resistor.
(A) Stainless steel (B) Nichrome (C) Copper (D) Bronze
48. The right hand thumb rule is also called rule……………
(A) Newton’s law of motion (B) Newland’s law of Octave
(C) Mendeleev’s periodic law (D) Maxwell’s cork- screw
49. …………. is used for electrical measurements.
(A) Thermometer (B) Galvanometer (C) Voltmeter (D) Electric meter
50. Which of the following scientist invented the rule of electromagnetic induction?
(A) Newton (B) Kepler (C) Mendeleev (D) Michael Faraday
51. Which of the following substance contracts on heating?
(A) Lukewarm water (B) Ice (C) Iron (D) Mercury
52. If pressure increases, the melting point of a substance ………………
(A) does not change (B) decreases (C) increases (D) remains constant
53. The vapour content in the air is measured by ………………
(A) relative humidity (B) dew point (C) absolute humidity (D) none of these
54. Humid and dry nature of air depends on the …………….
(A) amount of vapor in the air (B ) amount of vapor to make the air saturated
(C) temperature of the air (D) all of the above
55. Vapours in air condenses to form ………….
(A) fog (B) snowfall (C) rainfall (D) (A), (B) and (C)
56. When the temperature of water decreases below 4 °C it’s volume ………………
(A) decreases (B) increases (C) remains same (D) none of these
57. In a region with a cold climate the aquatic animals can survive at 4 °C, because …………..
(A) ice floating on water is insulator
(B) the heat from water cannot transfer to the atmosphere
(C) anomalous behaviour of water
(D) all the above
58. From the options given below the specific heat of …………… is maximum.
(A) copper (B) silver (C) iron (D) mercury
59. Ice-ball is prepared from shredded ice again. This is the example of …………..
(A) melting (B) condensation (C) regelation (D) freezing
60. The SI unit of specific heat is ………………
(A) kcal (B) cal (C) cal/g °C (D) J/kg °C
61. ………… apparatus is used to study the anomalous behaviour of water.
(A) calorimeter (B) Joule’s apparatus (C) Hope’s apparatus (D) Thermos flask
62. ………….. heat is necessary to raise 1 kg of water from 14.5 °C to 15.5 °C.
(A) 4180 joule (B) 1 kjoule (C) calorie (D) 4180 calorie
63 Due to …………….. pencil looks bent in water, in the given experiment.

(A) refraction of light
(B) dispersion of light
(C) internal reflection of light
(D) reflection of light
64. In the following diagram if ∠i = 40°, then ∠e = ?
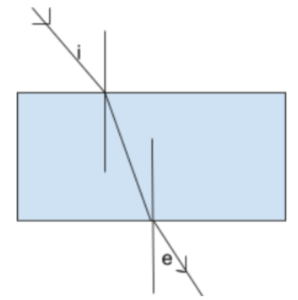
(A) 50° (B) 40° (C) 60° (D) 90°
65. A ray of light strikes the glass slab at an angle 40° with the surface of the slab. Then the angle of incidence will be ……………… °.
(A) 50 (B) 40 (C) 60 (D) 90
66. We see the sun even after it goes below the horizon, because …………….
(A) refraction of light (B) dispersion of light (C) partial reflection of light (D) reflection of light
67. ……….. is the unit of refractive index.
(A) cm (B) m (C) degree (D) refractive index has no unit
68. n = ……… This law is also called as Snell’s law.
(A) sin r/sin i (B) sin r/sin e (C) sin e/sin r (D) sin i/sin r
69. Lights of different colours are used as signal for safety transport. From these, the wavelength of red light is …………… nm.
(A) 400 (B) 500 (C) 600 (D) 700
70. If the refractive index of air with respect to glass is 2/3 .What is the refractive index of glass with respect to air?
(A) 2/3 (B) 3/2 (C) 1/3 (D) 1/2
71. The process of separation of light into its component colours while passing through a medium is called …………
(A) reflection of light (B) refraction of light (C) dispersion of light (D) absorption of light
72. Light changes its direction when going from one transparent medium to another transparent medium. This is called …………..
(A) reflection of light (B) refraction of light (C) dispersion of light (D) absorption of light
73. A ray of light gets refracted ……………. while entering the lens.
(A) once (B) twice (C) thrice (D) doesn’t happen
74. The point inside the lens on the principal axis through which light rays pass without changing their path is called ………..
(A) centre of curvature (B) optical centre (C) principal focus (D) axiom point
75. Virtual image is formed by a convex lens if an object is placed …………………
(A) at infinity (B) at 2F1 (C) at focus F1 (D) between F1 and O
76. In the convex lens if an object is placed at 2F1, the image is formed at ………….
(A) F1 (B) 2F1 (C) beyond 2 F1 (D) On the same side of the lens as the object
77. All distances parallel to the principal axis are measured from the …………..
(A) optical centre (B) centre of curvature (C) principal focus (D) infinity
78. A small hole of changing diameter at the centre of Iris is called …………….
(A) optic nerves (B) cornea (C) optic disc (D) pupil
79. For a normal human eye the near point is at ………….
(A) 2.1cm (B) 2.5 cm (C) 25 cm (D) 5 cm
80. The image formed by …………. lens is always virtual and small.
(A) plane convex (B) biconvex (C) biconcave (D) bifocal
81. In a relaxed state, the focal length of healthy eyes is …………..
(A) 2 cm (B) 2.5 cm (C) 25 cm (D) 5 cm
82. For a specific glass lens f = 0.5 m. This is the only information given to the student. Which type of lens is given to him and what is its power?
(A) power 2 D; convex lens (B) power 1 D; concave lens (C) power -0.5 D; concave lens (D) power -0.25 D; convex lens
83. In Myopia the human eye ………..
(A) cannot see nearby objects distinctly
(B) cannot see distant objects clearly
(C) cannot see nearby as well as distant objects clearly
(D) can see nearby as well as distant objects clearly
84. Due to elongation of …………….. and increase in curvature of the eye lens, a person cannot see distant objects clearly.
(A) eyeball (B) pupil (C) eyelid (D) cornea
85. In hypermetropia human eye ……………..
(A) can see distant objects clearly
(B) can see nearby objects distinctly
(C) cannot see nearby as well as distant objects clearly
(D) can see nearby as well as distant objects clearly
86. Bifocal lens is required to correct ………………. defect.
(A) myopia (B) hypermetropia (C) presbyopia (D) none of these
87. …………….. times larger images can be obtained by using a simple microscope.
(A) 5 (B) 10 (C) 20 (D) 60
88. ………….. is a combination of two convex lenses with small focal length.
(A) simple microscope (B) compound microscope (C) telescope (D) none of these
89. Bronze is an alloy of ………..
(A) copper and tin (B) copper and zinc (C) copper and iron (D) iron and nickel
90. ………….. is an alloy made from iron, carbon and chromium.
(A) brass (B) bronze (C) stainless steel (D) amalgam
91. …………. is basic oxide.
(A) CO2 (B) K2O (C) SO2 (D) Al2O3
92. In electrolytic reduction of alumina is ………….. used as a cathode.
(A) sulphur (B) graphite (C) platinum (D) aluminium
93. Iron is ………….
(A) more reactive than zinc (B) more reactive than aluminium (C) less reactive than copper (D) less reactive than aluminium
94. If Cu, Fe, Zn, Al elements are arranged in increasing order of their reactivity then the correct order would be which of the following?
(A) Cu, Fe, Zn, Al (B) Al, Cu, Fe, Zn (C) Zn, Al, Cu, Fe (D) Fe, Zn, Al, Cu
95. Which of the following method is used to prevent the accumulation of greenish layer on brass due to corrosion?
(A) electroplating (B) anodization (C) tinning (D) alloying
96. In Wilfley table method to separate particles of gangue ………………… method is used.
(A) Magnetic (B) Froth floatation (C) Leaching (D) gravitation
97. Aluminium oxide is oxide ………….
(A) acidic (B) basic (C) neutral (D) amphoteric
98. Atomic number of aluminium is …………… and its electronic configuration is ……………
(A) 13, (2,8,3) (B) 12(2,8,2) (C) 13,(3,10) (D) 12,(2,10)
99. The chemical formula of zinc blend is ………….
(A) ZnSO4 (B) ZnS (C) ZnCO3 (D) ZnO
100. Extraction of moderately reactive elements is done by …………….. and …………….. method.
(A) roasting and calcination (B) roasting and reduction (C) separation and calcination (D) none of these
101. Corrosion of silver causes a black layer of ……………….
(A) Silver nitrate (B) silver oxide (C) silver sulphide (D) silver carbonate
102. To prevent corrosion of iron and steel …………….. method is used.
(A) electroplating (B) anodization (C) tinning (D) galvanizing
103. In preparation of Aqua regia hydrochloric acid and …………… acid are mixed.
(A) sulphuric acid (B) nitric acid (C) carbonic acid (D) phosphoric acid
104. The sound of one metal colliding with another makes a noise, this property is called as …………….
(A) good conductors (B) ductility (C) sonority (D) malleability
105. ……………. exist in a liquid state at room temperature.
(A) Chlorine (B) Bromine (C) Iodine (D) Fluorine
106. Ionic compounds are electrically …………..
(A) positively charged (B) negatively charged (C) neutral (D) conductor
107. ……………. is good conductor of heat but bad conductor of electricity.
(A) graphite (B) diamond (C) coal (D) iodine
108. …………… is the least reactive metal.
(A) silver (B) sodium (C) zinc (D) gold
109. …………. forms a green colour in the water.
(A) CuS04 (B) FeS04 (C) NaCl (D) all the above
110. Stainless steel is an alloy of ……………
(A) copper (B) tin (C) zinc (D) iron
111. When one of the metals in an alloy is mercury the alloy is called …………..
(A) amalgam (B) sodium amalgam (C) zinc amalgam (D) all the above
112. The minerals from which the metal can be separated economically are called ……………
(A) minerals (B) ores (C) gangue (D) alloy
113. Generally the melting and boiling point of carbon compounds are found to be less than ……………… °C.
(A) 300 (B) 100 (C) 200 (D) 150
114. Number of valence electrons in a carbon atom is ………….
(A) 4 (B) 5 (C) 1 (D) 3
115. The bond between two oxygen atoms is bond ………..
(A) double (B) triple (C) single (D) none of these
116. The molecular masses of a carbon compound spread over a range of …………..
(A) 1012 (B) 1014 (C) 1010 (D) 1013
117. The unsaturated hydrocarbons containing a carbon-carbon double bond are called ……………..
(A) Alkenes (B) Alkanes (C) Alkynes (D) Alcohol
118. The unsaturated hydrocarbons whose structures contain a carbon-carbon triple bond are called ………………
(A) Alkenes (B) Alkanes (C) Alkynes (D) Alcohol
119. The phenomenon in which compounds having different structural formulae have the same molecular formula is called ……..
(A) structural isomerism (B) catenation (C) homologous (D) functional group
120. From the following hydrocarbon …………… is the cyclic hydrocarbon.
(A) isobutane (B) propyne (C) benzene (D) isobutylene
121. While going in an increasing order there is a rise in the molecular mass of the consecutive members of the homologous series by ……….
(A) 14 u (B) 15u (C) 16 u (D) 17u
122. The general molecular formula for the homologous series of alkynes is ……………..
(A) CnH2n (B) CnH2n+2 (C) CnH2n -2 (D) CnH2n – 1
123. ……………. is one of the combustible components of L.P.G.
(A) Methane (B) Ethane (C) Propane (D) Butanol
124. At room temperature ethanol is ……………..
(A) solid (B) gas (C) plasma (D) liquid
125. Generally …………. is called spirit.
(A) methanol (B) ethanol (C) propanol (D) butanol
126. Due to …………………, we can gather information about worldwide events sitting at home.
(A) worldwide web (B) internet (C) artificial satellite (D) natural satellite
127. The first person to step on the moon is ………….
(A) Neil Armstrong (B) Rakesh Sharma (C) Kalpana Chawla (D) Sunita Williams
128. The first artificial satellite was sent to space by Soviet Union in 1957.
(A) Apollo (B) Chandrayaan (C) Sputnik (D) Luna 2
129. If a spacecraft is to be sent to travel to outer space, it must have minimum velocity of ………….
(A) 11.2 km/s (B) 11.6km/s (C) 13.2 km/s (D) 1.4 m/s
130. A group of students from COEP Pune sent a small satellite ……………. through ISRO in 2016.
(A) Luna 6 (B) Apollo 6 (C) Swayam (D) Param
131. The astronomical object closest to us is the ……………
(A) Moon (B) Mars (C) Saturn (D) Mercury