1. Element A and B follows the Newland’s octaves rule. How many elements are there in between A and B?
Ans: Element A and B follows the Newland’s octaves rule and six elements are there in between A and B.
2. Write the characteristic of Dobereiner’s triads?
Ans: In each triad, the elements were placed according to increasing order of their atomic masses and, the atomic mass of the middle clement in each triad was approximately equal to the mean of the atomic masses of the other two elements.
3. Up to which element does Newland’s octave rule apply?
Ans: Newland’s octave rule is applicable only up to calcium.
4. Write the molecular formula of the oxide of any one of the elements in Mendeleev’s periodic table.
Ans: Element Na Molecular formula Na2O
5. Write the name of noble gas having 2 electrons in its valence shell.
Ans: The noble gas having 2 electrons in its valence shell is helium.
6. Write the name of an element having electronic configuration 2,8,2.
Ans: Magnesium (Mg) is an element having electronic configuration 2,8,2.
7. Which two elements show an ambiguity regarding their sequence in Mendeleev’s periodic table?
Ans: Cobalt (59Co) and nickel (59Ni) show an ambiguity regarding their sequence in Mendeleev’s periodic table.
8. The elements beryllium, magnesium and calcium are in group 2. What will be their valency?
Ans: The elements beryllium, magnesium and calcium are in group 2 and their valency is 2.
9. The modern periodic table is divided into which blocks?
Ans: The modem periodic table is divided into s-block, p-block, d-block, f-block.
10. What determines the chemical reactivity of elements?
Ans: The chemical reactivity of an element is determined by the number of valence electrons in it and the shell number of the valence shell.
11. Write chemical formula for rust.
Ans: Chemical formula for rust: Fe2O3. xH2O
12. Complete the given chemical reaction.
CuSO4(aq)+ Fe(s) → ______ + _______
Ans: CuSO4(aq)+ Fe(s) → FeSO4(aq) + Cu(s)
13. What is the heating effect of electric current?
Ans: When a resistor is connected in an electrical circuit, heat is produced in it due to the current. This is known as the heating effect of current.
14. Which metal is used to make the filament of an electric bulb?
Ans: Tungsten metal is used to make the filament of an electric bulb.
15. What is a short circuit?
Ans: Due to a fault in the equipment or if the plastic coating on the ‘live’ and the ‘neutral’ wires gives away the two wires come in contact with each other and a large current flows through it producing heat. This is known as short circuit.
16. What is the potential difference? How much is the potential difference between live and neutral wires?
Ans: The potential difference is the difference in the electric potential of two different points. The potential difference between live and neutral wires is around 220 V.
17. What is overloading?
Ans: When consumption of electrical power is huge, excessive current is drawn from the transformer supplying the electricity, and if the capacity of the transformer is insufficient, its fuse wire melts and the supply gets shutdown. Such events as called as overloading.
18. What is used to turn off the sudden increase in current in the electrical circuit of the house nowadays?
Ans: Nowadays, Miniature Circuit Breakers (MCB) is used to turn off the sudden increase in current in the electrical circuit of the house.
19. Write two devices based on the heating effect of electric current.
Ans: Electric iron, electric bulb.
20. Write Fleming’s right hand thumb rule.
Ans: If a current carrying straight conductor is held in our right hand such that the thumb points towards the direction of current, then the curled fingers around the conductor will give the direction of the magnetic field.
21. Write Fleming’s left hand rule.
Ans: Stretch the index finger, the middle finger and the thumb of the left hand mutually perpendicular to each other. If the index finger is in the direction of the magnetic field and the middle finger points in the direction of the current, then the thumb will point towards the direction of the force on the conductor.
22. Write Fleming’s right hand rule.
Ans: Stretch the thumb, index finger and middle finger of the right hand so that they are perpendicular to each other. If the index finger indicates the direction of magnetic field and the thumb shows the direction of motion of the conductor, then the middle finger will show the direction of induced current.
23. What is a solenoid?
Ans: When a copper wire with a resistive coating is wound in a chain of loops (like a spring), it is called as solenoid.
24. Label the following two diagrams.
i. Right hand thumb rule.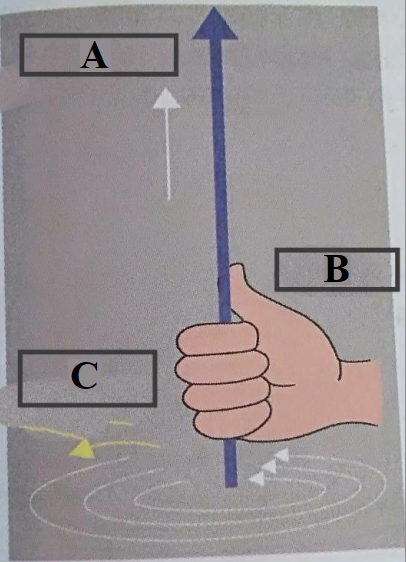
Ans:
Label A : Direction of Electric current
Label B : Right hand
Label C : Magnetic field
ii. Fleming’s right hand rule.
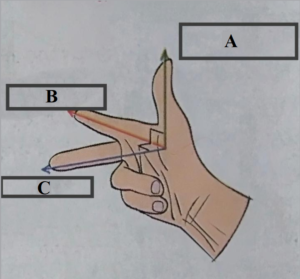
Ans:
Label A : Motion of the conductor
Label B : Direction of the magnetic field
Label C : Direction of induced current
25. Define boiling point of a liquid.
Ans: The constant temperature at which liquid converts into gas is called its boiling point.
26. What is meant by regelation?
Ans: The phenomenon in which the ice converts to liquid due to applied pressure and then re-converts to ice once the pressure is removed is called regelation.
27. How fog is formed?
Ans: When water vapour condenses around microscopic solid particles such as dust, fog is formed.
28. What is a dew point temperature?
Ans: When unsaturated air (of specific volume) at a certain temperature is taken and its temperature is decreased, a temperature is reached at which the air becomes saturated with vapour. This temperature is called the dew point temperature.
29. What does the existence of drops of w ater on the leaves of a tree in the morning indicate?
Ans: Existence of drops of water on leaves in the morning indicates that air is saturated with vapour.
30. Which temperature segment is chosen when determining the unit of heat? Why?
Ans: While deciding the unit for heat, temperature interval chosen is 14.5 °C to 15.5 °C.
Reason:
When 1 kg of water is heated through 1 °C in various temperature ranges, the amount of heat required to raise temperature by 1 °C varies slightly from range to range.
Hence, it is essential to define a specific temperature range while defining unit of heat.
31. Identify the wrong figure from the following.
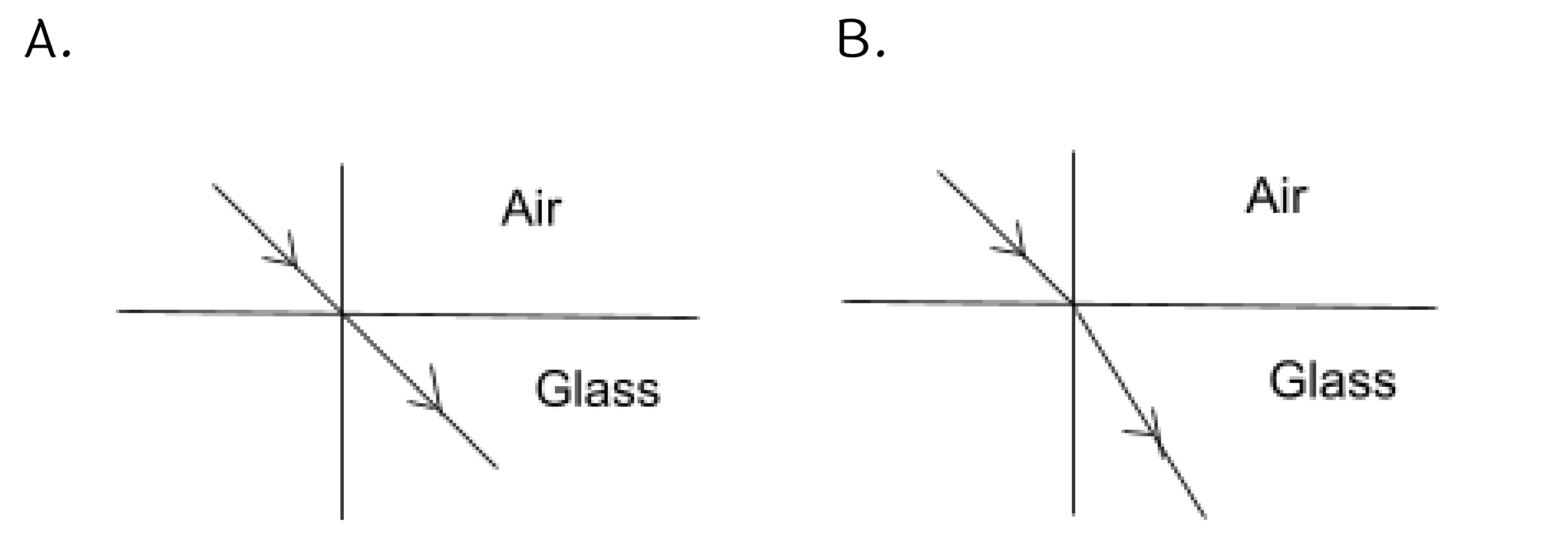
Ans: Figure (A) is the wrong figure.
32. Write the molecular formula of the given compound.
1. Ethyl ethanoate
2. Sodium ethanoate
3. Sodium ethoxide
4. Stearic acid
5. Oleic acid
6. Palmitic acid
Ans:
1. Ethyl ethanoate: C4H8O2
2. Sodium ethanoate: C2H3O2Na
3. Sodium ethoxide: C2H5ONa
4. Stearic acid: C17H35COOH or C18H36O2
5. Oleic acid: C17H33COOH or C18H34O2
6. Palmitic acid: C15H31COOH or C16H32O2
33. Write the molecular formula of the given compound.
1. Ethylene
2. Benzene
3. Acetic acid
4. Propylene
5. Acetylene
6. Ethyl alcohol
7. Acetone
8. Propene
9. Ethanol
10. Ethanoic acid
11. Isobutane
Ans:
1. Ethylene: C2H4
2. Benzene: C6H6
3. Acetic acid: CH3COOH or C2H4O2
4. Propylene: C3H6
5. Acetylene: C2H2
6. Ethyl alcohol: C2H5OH or C2H6O
7. Acetone: C3H6O
8. Propene: C3H6
9. Ethanol: C2H5OH or C2H6O
10. Ethanoic acid: CH3COOH or C2H4O2
11. Isobutane: C4H10
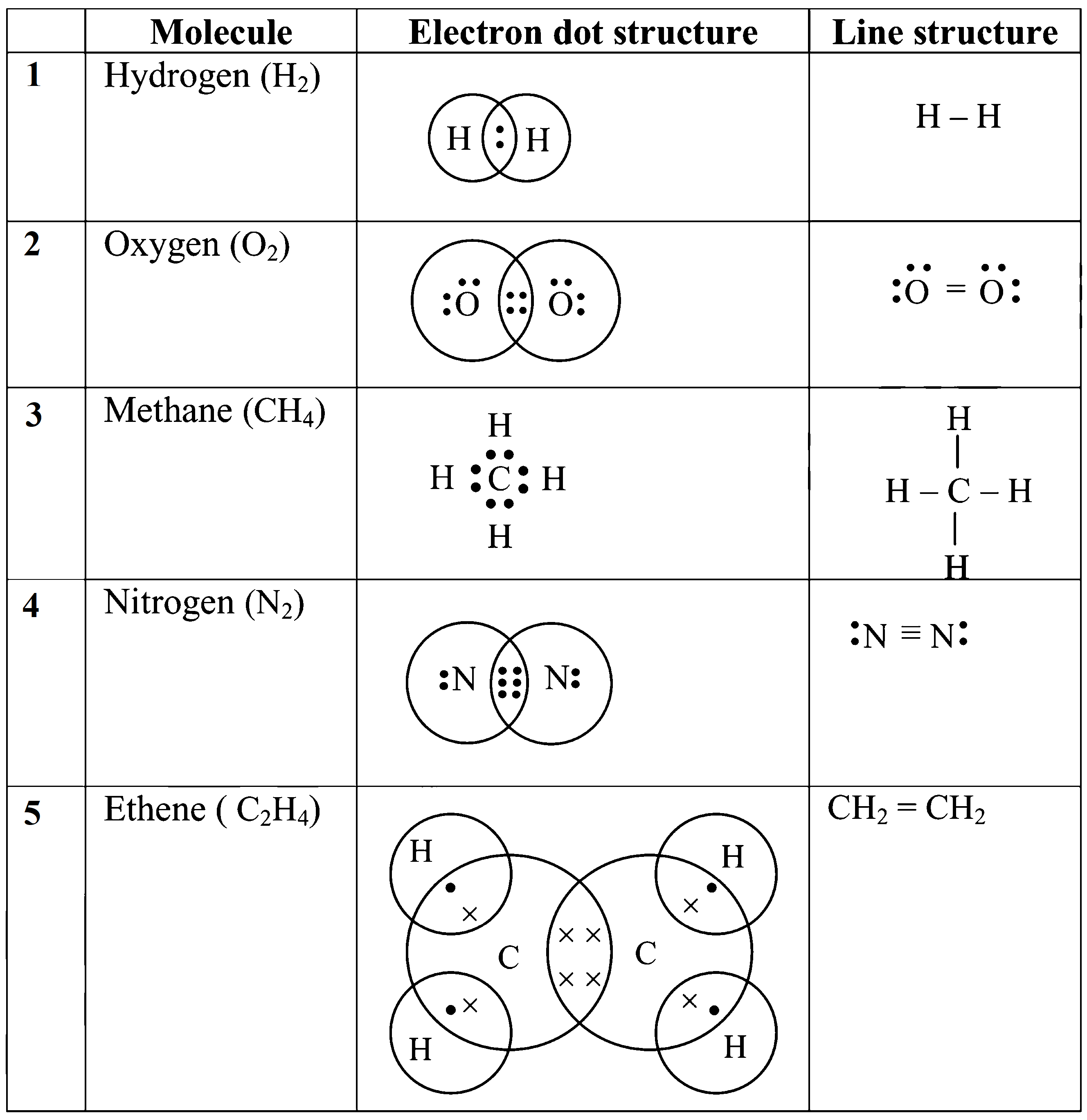
34. Draw electron dot structure and line structure for given molecules.
1. Hydrogen 2. Oxygen 3. Methane 4. Nitrogen 5. Ethene
Ans: