1. Fill in the blanks.
a. The number of molecules of water of crystallization in washing soda is …………….
b. The chemical name of baking soda is ……………
c. ………….. is used in treatment of hyperthyroidism.
d. The chemical name of Teflon is ………….
Ans.
a. The number of molecules of water of crystallization in washing soda is 10
b. The chemical name of baking soda is Sodium bicarbonate
c. Iodine -223 is used in treatment of hyperthyroidism.
d. The chemical name of Teflon is Polytetrafluoroethene (C2F4)n
2. Match the pairs
Group A
1. Saturated brine
2. Fused salt
3. CaOCl
4. NaHCO
Group B
a. sodium metal freed
b. basic salt
c. crystallization of salt
d. oxidation of colour
Ans.
Group A
1. Saturated brine
2. Fused salt
3. CaOCl
4. NaHCO
Group B
a. crystallization of salt
b. sodium metal freed
c. oxidation of colour
d. basic salt
3. Write answers to the following
a. What is meant by radioactivity?
Ans.
Elements with a high atomic number such as uranium, thorium, radium have a property of spontaneously emitting invisible, highly penetrating and high energy radiation. This property is called radioactivity.
b. When is said to be the nucleus unstable?
Ans.
Nucleus is said to be unstable when the element has the radioactive property as the radiation occurs from the unstable nuclei only.
c. Which diseases are caused by artificial food colours ?
Ans.
Diseases like ADHD (Attention Deficit Hyperactivity Disorder) can affect children due to excessive consumption of foods with added food colours.
d. Where in the industrial field is radioactivity used?
Ans.
Radiography – Internal cracks and voids in cast iron articles and iron solder can be detected with the help of gramma rays by the radiography camera. This technique is used for detecting flaws in metal work.
Measurement of thickness, density and level – It is necessary to maintain the required thickness in the manufacture of aluminium, plastic, iron sheets of differing thickness. In the manufacturing process, a radioactive substance is placed on one side and an instrument to measure radiation on the other. The radiation read by the measuring instrument varies with the thickness of the sheet.
Luminescent paint and radioluminescence – The radioactive substances radium, promethium, tritium with some phosphor are used to make certain objects visible in the dark, for example, the hands of a clock, and certain other objects.
Use in Ceramic articles – Luminous colours are used to decorate ceramic tiles, utensils, plates, etc.
e. Write down properties of teflon.
Ans.
1. The atmosphere and chemical substances have no effect on Teflon. 2. Neither water nor oil will stick to Teflon coated articles. 3.High temperatures do not affect Teflon as its melting point is 327°C. 4.Teflon coated articles are easy to clean.
f. What type of colours will you use to celebrate ecofriendly Rang Panchami? Why?
Ans.
(1) We should use natural colours to celebrate ecofriendly rang panchami. Because natural colors saves our skins and our environment thus conserve our biodiversity. (2) When these colors percolate into the soil and water they do not add toxicity and cause no harm to the life forms that live in the soil and water.
g. Why has the use of methods like Teflon coating become more common?
Ans.
The use of methods like Teflon coating became more common because of following properties of teflon:
(1) The atmosphere and chemical substances have no effect on Teflon. (2) Neither water nor oil will stick to Teflon coated articles. (3) High temperatures do not affect Teflon as its melting point is 327°C. (4) Teflon coated articles are easy to clean.
4. Give scientific explanation
a. Bleaching powder has the odour of chlorine.
Ans.
(1) Bleaching powder undergoes slow decomposition due to the carbon dioxide in air and chlorine gas is released. (2) Bleaching powder gets its property because of this release of chlorine gas and hence has the odour of chlorine. 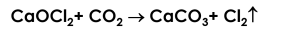
b. The hard water of a well becomes soft on adding washing soda to it.
Ans.
(1) The hardness of water is due to the presence of chlorides and sulphates of calcium and magnesium in it. (2) When washing soda is added, it soften such water and make it suitable for use. (3) The reaction with washing soda, causes the formation of insoluble carbonate salts of magnesium and calcium.
c. Soap forms a precipitate in hard water.
Ans. (1) When soap is mixed with hard water, calcium and magnesium salts of fatty acids are formed. (2) These being water insoluble they form a precipitate and that is why lather is not formed.
d. The particles of powder are given an electric charge while spraying them to form the powder coating.
Ans. (1) The particles of the powder are given an electrostatic charge due to which a uniform layer of the powder sticks to the metal surface. (2) Then the object is heated in the over along with the coating. (3) A chemical reaction occurs in the layer, resulting in the formation of long cross-linked polymeric chains. (4) This powder coating is highly durable, hard and attractive.
e. The aluminium article is used as an anode in the anodising process.
Ans. (1) Dilute acid is taken in the electrolytic cell and the aluminium article is dipped in it as the anode. (2) When an electric current is passed hydrogen gas is released at the cathode and oxygen gas at the anode. (3) A reaction with oxygen occurs and a layer of hydrated aluminium oxide is formed on the anode, i.e. the iron article. (4) This layer can be made attractive by adding colour in the cell during electrolysis.
f. When the radiation coming out from certain radioactive substance is passed through an electric field, marks are found at three places on the photographic plate placed in its path.
Ans. (1) Because when the rays were allowed to pass through an electrical field and a photographic plate was held in their path. It was found that the radiation was divided into three types. (2) One type of radiation deviated slightly towards the negatively charged plate, while the second type of radiation deviated substantially towards the positively charged plate. (3) However, the third type of radiation did not deviate at all in the electrical field. (4) The rays which deviated slightly toward negatively charged plate are called alpha rays, those which deviate substantially towards the positively charged plate are called beta rays and those which do not deviate at all are called gamma rays.
g. A certain type of ceramic tiles are fixed on the outer layer of a space shuttle.
Ans. (1) Ceramics can withstand high temperatures without decomposing. (2) Ceramic is brittle, water resistant and an electrical insulator. (3) It is used in electrical instruments, for coating the interior of a kiln, the outer surfaces of ships and blades of jet engines. (4) Thus, a certain type of ceramic tiles are fixed on the outer layer of a space shuttle.
5. Write answers to the following
a. Write about artificial food colours, the substances used in them and their harmful effects.
Ans. (1) Food colours are mixed in most soft drinks and foodstuffs available in the market. (2) These food colours are in the form of powders, gels and pastes. (3) Food colours are used in domestic as well as commercial products. (4) Certain colours and essences are added to ice cream, ice candies, sauce, fruit juices, cold drinks, pickles, jams and jelly. (5) Food colours are often found to be added to packaged meat (chicken, mutton), chilli powder, turmeric, sweets and other similar substances so as to give them a good colour. (6) Food colours are natural as well as artificial. The food colours prepared from seeds, beetroot, flowers and fruit concentrate are natural. Tetrazene, sunset yellow are artificial food colours used extensively.
Harmful effects of artificial food colours
(1) Food colours added to pickles, jam and sauce contain small quantities of lead and mercury. These can be harmful for those who consume these products on a regular basis. (2) Diseases like ADHD (Attention Deficit Hyperactivity Disorder) can affect children due to excessive consumption of foods with added food colours.
b. What is meant by water of crystallization? Give examples of salts with water of crystallization, and their uses.
Ans.
Water of crystallization is the fixed number of water molecules present in one formula unit of a salt.
Examples of salts with water of crystallization
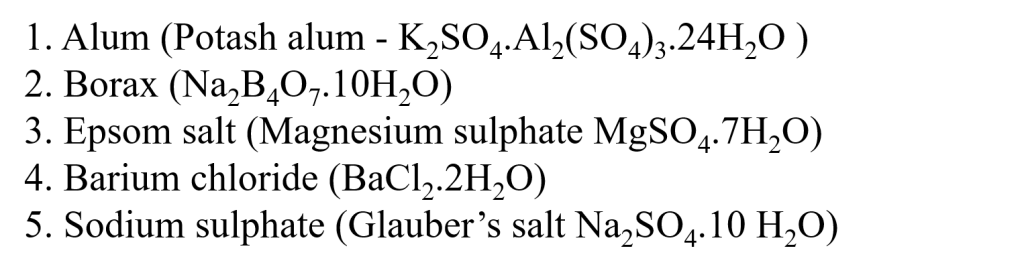
Uses of salts
1. Alum is used in the process of water purification.
2. Borax can kill insects such as ants. It is used in laundry detergents and household cleansers to help whiten and get rid of dirt.
3. Epsom salt Used to soften skin, soothe tired feet, and relieve muscle aches.
4. Barium chloride is used in the purification of brine solution in caustic chlorine plants.
5. Sodium sulphate is used to dry organic liquids.
c. Write briefly about the three methods of electrolysis of sodium chloride.
Ans.
(1) When an electric current is passed through a saturated solution of sodium chloride (brine) it is electrolysed and hydrogen gas is released at the cathode while chlorine gas is released at the anode. This
method is used for production of chlorine gas. In this method an important basic compound NaOH is formed in the cell. 
(2) When salt is heated to a high temperature (about 800°C) it melts. This is called the fused state of the salt.
(3) When fused salt is electrolysed, chlorine gas is released at the anode and liquid sodium metal, at the cathode.
6. Write the uses.
a. Anodizing
Ans.
(1) Anodized cooking utensils like griddles, cookers and pans are non- stick, scratch resistant and can be easy cleaned. (2) Anodized utensils are heat resistant and long-lasting.
b. Powder coating
Ans.
(1) Powder coating is used as applying a layer harder than paint on the surface of an iron object to prevent rusting. (2) Used on Plastic and Medium density fibre (MDF) board, to make them highly durable, hard and attractive.
c. Radioactive substances
Ans.
(1) (a) Radiography, (b) Measurement of thickness, density and level, (c) luminescent paint and radioluminescence, (d) Use in Ceramic articles
(2) Field of agriculture: (a) The radioactive isotope cobalt-60 is used for food preservation. (b) Onions, potatoes are irradiated with gamma rays from cobalt-60 to prevent their sprouting.
(3) Medical science: (a) Polycythemia (b) Bone cancer (c) Hyperthyroidism (d) Tumour detection.
d. Ceramic
Ans.
(1) It is used in electrical instruments, for coating the interior of a kiln, the outer surfaces of ships and blades of jet engines. (2) They are used to make pottery, bricks, tiles, cements, and glass. (3) Ceramics are also used at many places in gas turbine engines.
7. Write the harmful effects
a. Artificial dye
Ans. (1) Dyeing hair can have adverse effects like hair fall, damage to hair texture, burning of skin, adverse effect on eyes, etc. (2) Lipstick contains a dye named carmine. It does not affect lips but causes stomach disorders. (3) Excessive use of plants for making natural dyes results in deterioration of the environment.
b. Artificial food colour
Ans.
(1) Food colours added to pickles, jams and sauces contain small quantities of lead and mercury. These can be harmful for those who consume these products on a regular basis. (2) Diseases like ADHD Attention Deficit Hyperactivity Disorder) can affect children due to excessive consumption of foods with added food colours.
c. Radioactive substances
Ans.
(1) The central nervous system is affected by radioactive radiations. (2) Hereditary defects are generated by bombardment of radiation on D.N.A in the body. (3) Radioactive radiation can penetrate the skin, and causes diseases like skin cancer, leukemia. (4) The radiative pollutants created due to explosions enter the body through air and it is difficult to control them. (5) The radioactive pollutants released in the sea enter the bodies of fishes and through them enter the human body.
d. Deodorant
Ans.
(1) Aluminium – Zirconium compounds are the most harmful chemicals in the deodorant. Disorders like headache, asthma, respiratory disorders, heart disease are likely to occur without our knowledge.
(2) There is a possibility of various skin disorders and also skin cancer due to the aluminium chlorohydrates.
8. Write the chemical formula
Bleaching powder, common salt, baking soda, washing soda 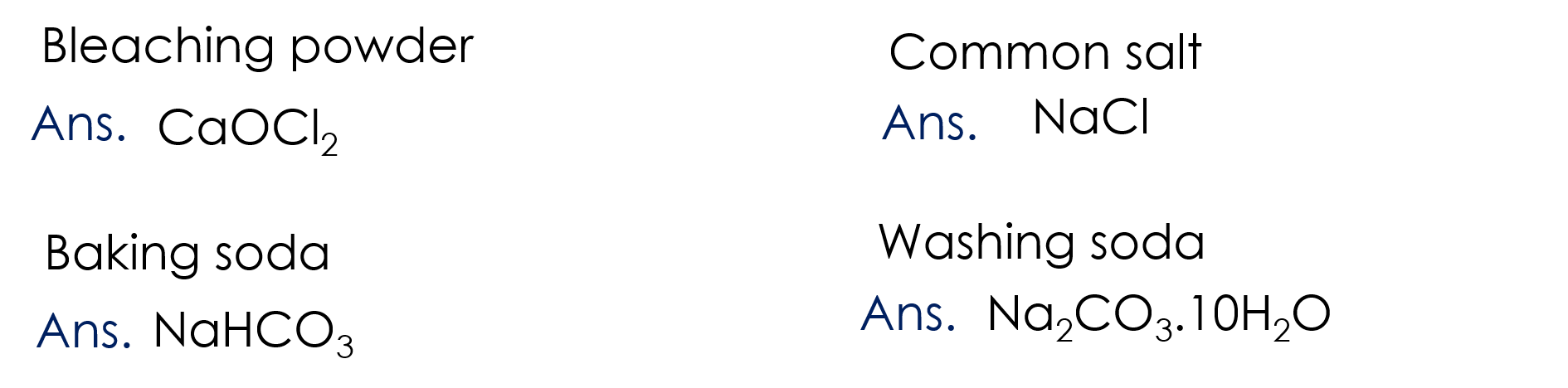
9. Explain what you see in the following picture
Ans.
The picture depicts of the powder coating i.e. powder is sprayed on the polished metal surface by electrostatic spray deposition. Powder coating is a type of coating that is applied as a free-flowing, dry powder. The coating is typically applied electrostatically and is then cured under heat to allow it to flow and form a skin. The powder may be a thermoplastic or a thermoset polymer. It is usually used to create a hard finish that is tougher than conventional paint. Powder coating is mainly used for coating of metals, such as household appliances, aluminum extrusions, drum hardware and automobile and bicycle parts.